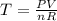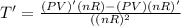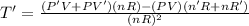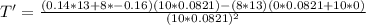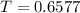Mathematics, 06.10.2019 01:00 Jsanders2276
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), and volume v (in liters) is pv = nrt, where n is the number of moles of the gas and r = 0.0821 is the gas constant. suppose that, at a certain instant, p = 8.0 atm and is increasing at a rate of 0.14 atm/min and v = 13 l and is decreasing at a rate of 0.16 l/min. find the rate of change of t with respect to time at that instant if n = 10 mol. (round your answer to four decimal places.)
 = k/min
= k/min

Answers: 3


Another question on Mathematics

Mathematics, 20.06.2019 18:04
Approximately how many more job openings are expected for security guards than pharmacy technicians? a. 2,000 b. 11,100 c. 21,000 d. 32,000
Answers: 1

Mathematics, 21.06.2019 18:30
1.two more than the quotient of a number and 6 is equal to 7 use the c for the unknown number translate the sentence into an equation
Answers: 1


Mathematics, 21.06.2019 23:20
Identify the function that contains the data in the following table: x -2 0 2 3 5 f(x) 5 3 1 2 4 possible answers: f(x) = |x| + 1 f(x) = |x - 2| f(x) = |x - 2| - 1 f(x) = |x - 2| + 1
Answers: 1
You know the right answer?
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), an...
Questions

History, 08.10.2019 02:30

History, 08.10.2019 02:30

History, 08.10.2019 02:30


Geography, 08.10.2019 02:30




History, 08.10.2019 02:30

Chemistry, 08.10.2019 02:30




Mathematics, 08.10.2019 02:30



Social Studies, 08.10.2019 02:30


Mathematics, 08.10.2019 02:30

History, 08.10.2019 02:30