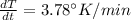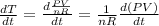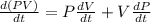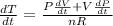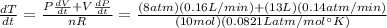Mathematics, 06.10.2019 10:00 mylanag12
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), and volume v (in liters) is pv = nrt, where n is the number of moles of the gas and r = 0.0821 is the gas constant. suppose that, at a certain instant, p = 8.0 atm and is increasing at a rate of 0.14 atm/min and v = 13 l and is decreasing at a rate of 0.16 l/min. find the rate of change of t with respect to time at that instant if n = 10 mol. (round your answer to four decimal places.)dt/dt = k/min

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 14:20
Factor p 2 + 18p + 32. (p+ 4)(p + 8) (p + 2)p+ 16) (p + 16)2
Answers: 1

Mathematics, 21.06.2019 16:20
The lengths of nails produced in a factory are normally distributed with a mean of 4.91 centimeters and a standard deviation of 0.05 centimeters. find the two lengths that separate the top 4% and the bottom 4%. these lengths could serve as limits used to identify which nails should be rejected. round your answer to the nearest hundredth, if necessary.
Answers: 3

Mathematics, 21.06.2019 20:30
Carley bought a jacket that was discounted 10% off the original price. the expression below represents the discounted price in dollars, based on x, the original price of the jacket.
Answers: 1

Mathematics, 21.06.2019 23:30
Drag each number to the correct location on the statements. not all numbers will be used. consider the sequence below. -34, -21, -8, 5, complete the recursively defined function to describe this sequence
Answers: 1
You know the right answer?
The gas law for an ideal gas at absolute temperature t (in kelvins), pressure p (in atmospheres), an...
Questions


English, 03.04.2020 07:58


Mathematics, 03.04.2020 07:58

Business, 03.04.2020 07:58

Mathematics, 03.04.2020 07:58




Biology, 03.04.2020 07:58

Mathematics, 03.04.2020 07:58

History, 03.04.2020 07:58


Advanced Placement (AP), 03.04.2020 07:58

Mathematics, 03.04.2020 07:58

Mathematics, 03.04.2020 07:58

Mathematics, 03.04.2020 07:58

English, 03.04.2020 07:58

History, 03.04.2020 07:58

Mathematics, 03.04.2020 07:58