
Mathematics, 24.12.2019 20:31 llamasking
50 pts and bra8inliest if u get it right and answer all
question 1 options:
indicate whether the reaction is an example of a synthesis, decomposition, combustion, single displacement or double displacement reaction. na3po4+3kohànaoh+k3po4
i think it might be double displacement but im not sure
question 2
name the reactants in the reaction from question 1. separate the reactants with a comma. do not use subscripts. for example, if the reactants are nh3 and h2o the answer would be entered as: nh3, h2o
i think its napo4 but im not sure
question 3
name the products in the reaction from question 1. separate the products with a comma. do not use subscripts. for example, if the products are nh3 and h2o the answer would be entered as: nh3, h2o
i think its naoh, k3po4 but im not sure
question 4
indicate whether the reaction is an example of a synthesis, decomposition, combustion, single displacement or double displacement reaction. c6h12+9o2à6co2+6h2o
question 5
name the reactants in the reaction from question 4. separate the reactants with a comma. do not use subscripts. for example, if the reactants are nh3 and h2o the answer would be entered as: nh3, h2o
i think its c6h12o6, 6o2 but im not sure
question 6
name the products in the reaction from question 4. separate the products with a comma. do not use subscripts. for example, if the products are nh3 and h2o the answer would be entered as: nh3, h2o
i think its 6co2,6h2o but im not sure
question 7
indicate whether the reaction is an example of a synthesis, decomposition, combustion, single displacement or double displacement reaction. pb+feso4àpbso4+fe
question 8 (
indicate whether the reaction is an example of a synthesis, decomposition, combustion, single displacement or double displacement reaction. caco3àcao+co2
question 9
indicate whether the reaction is an example of a synthesis, decomposition, combustion, single displacement or double displacement reaction. p4+3o2à2p2o3
question 10
indicate whether the reaction is an example of a synthesis, decomposition, combustion, single displacement or double displacement reaction. c3h6o+4o2à3co2+3h2o
question 11
balance the following equation to satisfy the law of conservation of mass. nai + pb(so4)2 àpbi4+ na2so4 enter the coefficients in order from left to right, separated for a comma. if there is no coefficient, use a 1. example: h2+o2àh2o is balanced as 2h2+o2à2h2o enter the answer as 2,1,2 question 12
balance the following equation to satisfy the law of conservation of mass. fe(oh)3à fe2o3+ h2o enter the coefficients in order from left to right, separated for a comma. if there is no coefficient, use a 1. example: h2+o2àh2o is balanced as 2h2+o2à2h2o enter the answer as 2,1,2 question 13
5 points extra credit
balance the following equation to satisfy the law of conservation of mass. nh3+ o2à no+ h2o enter the coefficients in order from left to right, separated for a comma. if there is no coefficient, use a 1. example: h2+o2àh2o is balanced as 2h2+o2à2h2o enter the answer as 2,1,2 question 14
predict the products of the following reaction: nai+cacl2à separate the products with a comma. do not use subscripts. for example, if the products are nh3 and h2o the answer would be entered as: nh3, h2o
question 15
predict the products of the following reaction: li+ kno3à (lithium is above potassium on the activity series for metals) separate the products with a comma. do not use subscripts. for example, if the products are nh3 and h2o the answer would be entered as: nh3, h2o
question 16
5 points extra credit
predict the products of the following reaction: rbno2 +baco3à separate the products with a comma. do not use subscripts. for example, if the products are nh3 and h2o the answer would be entered as: nh3, h2o if needed for polyatomic ions, use parenthesis. example: al(no3)3 should be entered as al(no3)3
quick! i have 7 minutes left on the timer!

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 16:30
In the figure shown below, m < 40 and ab =8. which equation could be used to find x?
Answers: 2



Mathematics, 21.06.2019 21:30
Write an equation of the line that passes through the point (2, 3) and is perpendicular to the line x = -1. a) y = 1 b) y = 3 c) y = 0 eliminate d) y = -3
Answers: 1
You know the right answer?
50 pts and bra8inliest if u get it right and answer all
question 1 options:
indi...
question 1 options:
indi...
Questions




Biology, 10.07.2019 16:00



History, 10.07.2019 16:00

Mathematics, 10.07.2019 16:00


Mathematics, 10.07.2019 16:00




History, 10.07.2019 16:00


Mathematics, 10.07.2019 16:00




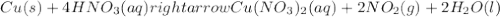 : oxidation reduction
: oxidation reduction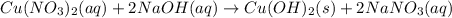 : precipitation
: precipitation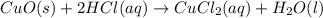 : Double displacement
: Double displacement

