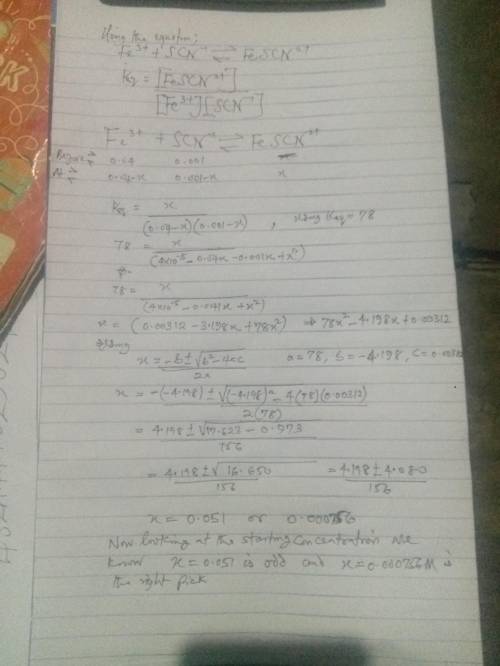Mathematics, 11.02.2020 21:27 Student2499
Using your calculated average value for Keq , calculate the SCN- concentration in a solution with an initial Fe3+ concentration of 4.00 x 10-2 M and the initial concentration of SCN- was 1.00 x 10-3 M. Is all the SCN- ion in the form of FeSCN2+? Hint: Set your product concentration to "x" and use the quadratic equation to solve. You will need to rearrange your Keq equation into the form of ax2 + bx + c = 0

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 17:00
Suppose a pendulum is l meters long. the time,t,in seconds that it tales to swing back and forth once is given by t=2.01
Answers: 1

Mathematics, 21.06.2019 17:40
Aregular hexagon has sides of 2 feet. what is the area of the hexagon? 12 ft2 12 ft2 24 ft2 6 ft2
Answers: 2


Mathematics, 21.06.2019 22:10
Aadc is formed by reflecting aabc across line segment ac, as shown in the figure. if the length of ac is 4 units, the area of aadc is square units.
Answers: 3
You know the right answer?
Using your calculated average value for Keq , calculate the SCN- concentration in a solution with an...
Questions


Biology, 23.11.2020 21:40

Health, 23.11.2020 21:40

Mathematics, 23.11.2020 21:40





French, 23.11.2020 21:40

English, 23.11.2020 21:40


English, 23.11.2020 21:40


Mathematics, 23.11.2020 21:40

English, 23.11.2020 21:40

Advanced Placement (AP), 23.11.2020 21:40



Chemistry, 23.11.2020 21:40