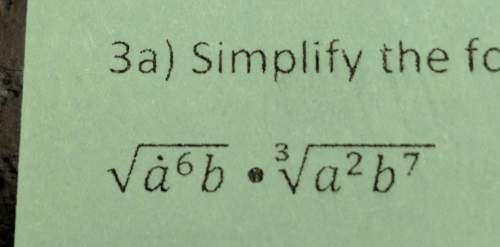Mathematics, 12.03.2020 19:36 naocarolina6
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), and volume V (in liters) is PV = nRT, where n is the number of moles of the gas and R = 0.0821 is the gas constant. Suppose that, at a certain instant, P = 9.0 atm and is increasing at a rate of 0.13 atm/min and V = 13 L and is decreasing at a rate of 0.16 L/min. Find the rate of change of T with respect to time at that instant if n = 10 mol. (Round your answer to four decimal places.)

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 16:40
What is the ratio of the change in y-values to the change in x-values for this function? a) 1: 13 b) 2: 5 c) 5: 2 d) 13: 1
Answers: 3

Mathematics, 21.06.2019 17:00
Find the measure of the interior angles of the following regular polygons: a triangle, a quadrilateral, a pentagon, an octagon, a decagon, a 30-gon, a 50-gon, and a 100-gon.
Answers: 2


You know the right answer?
The gas law for an ideal gas at absolute temperature T (in kelvins), pressure P (in atmospheres), an...
Questions



English, 10.02.2021 21:40


History, 10.02.2021 21:40


Mathematics, 10.02.2021 21:40

Biology, 10.02.2021 21:40






Mathematics, 10.02.2021 21:40

Physics, 10.02.2021 21:40

History, 10.02.2021 21:40



Chemistry, 10.02.2021 21:40

Mathematics, 10.02.2021 21:40