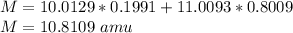Mathematics, 16.03.2020 23:47 oliviakate9230
Natural sample of boron consists of two isotopes. One has an exact mass of 10.0129 amu and its percent abundance is 19.91. The other isotope, of mass 11.0093 amu, has a percent abundance of 80.09. Calculate the average atomic mass.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 14:00
Do graduates from uf tend to have a higher income than students at fsu, five years after graduation? a random sample of 100 graduates was taken from both schools. let muf be the population mean salary at uf and let mufsu be the population mean salary at fsu. how should we write the alternative hypothesis?
Answers: 2

Mathematics, 21.06.2019 14:00
Which graph represents the solution of the inequality?
Answers: 1

Mathematics, 21.06.2019 19:30
He mass of a single atom of carbon can be found by dividing the atomic mass (12.01 g) by 6.022 x 10^23. which is the mass of a single carbon atom, correctly written in scientific notation with the correct number of significant figures?
Answers: 1

Mathematics, 21.06.2019 22:40
What rotation was applied to triangle def to create d’e’f’?
Answers: 2
You know the right answer?
Natural sample of boron consists of two isotopes. One has an exact mass of 10.0129 amu and its perce...
Questions

Mathematics, 24.10.2020 18:50

English, 24.10.2020 18:50



English, 24.10.2020 18:50

Mathematics, 24.10.2020 18:50

Mathematics, 24.10.2020 18:50


English, 24.10.2020 18:50

Mathematics, 24.10.2020 18:50


Mathematics, 24.10.2020 18:50


Mathematics, 24.10.2020 18:50

History, 24.10.2020 18:50

Mathematics, 24.10.2020 18:50

History, 24.10.2020 18:50


Biology, 24.10.2020 18:50

English, 24.10.2020 18:50