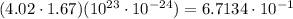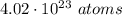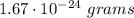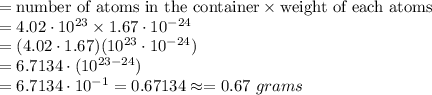Mathematics, 06.10.2019 07:01 ebt2367
Aclosed container has 4.02 ⋅ 1023 atoms of a gas. each atom of the gas weighs 1.67 ⋅ 10^−24 grams. which of the following shows and explains the approximate total mass, in grams, of all the atoms of the gas in the container?
6.71 grams, because (4.02 ⋅ 1.67) ⋅ (10^23 ⋅ 10^−24) = 6.7134
5.69 grams, because (4.02 + 1.67) ⋅ (10^23 ⋅ 10^−24) = 5.69
0.67 grams, because (4.02 ⋅ 1.67) ⋅ (10^23 ⋅ 10^−24) = 6.7134 ⋅ 10^−1
0.57 grams, because (4.02 + 1.67) ⋅ (10^23 ⋅ 10^−24) = 5.69 ⋅ 10^−1

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 18:40
Juliana says that she can use the patterns of equivalent ratios in the multiplication table below to write an infinite number of ratios that are equivalent to 6: 10. which statement explains whether juliana is correct? she is correct because she can multiply 6 and 10 by any number to form an equivalent ratio. she is correct because 6: 10 can be written as 1: 2 and there are an infinite number of ratios for 1: 2. she is not correct because the multiplication table does not include multiples of 10. she is not correct because 6: 10 is equivalent to 3: 5 and there are only 9 ratios in the multiplication table that are equivalent to 3: 5.
Answers: 1


Mathematics, 21.06.2019 23:30
When a valve is open 281 gallons of water go through it in one hour the number of gallons that would go through in 94 hours is
Answers: 1

Mathematics, 22.06.2019 03:00
An object is accelerating at a constant rate. its velocity in feet per second as a function of time in seconds can be modeled by the linear function v(t) = 2.5t. what does the dependent variable represent for this function? a) acceleration b) distance c) slope d) velocity
Answers: 3
You know the right answer?
Aclosed container has 4.02 ⋅ 1023 atoms of a gas. each atom of the gas weighs 1.67 ⋅ 10^−24 grams. w...
Questions

Social Studies, 13.12.2021 22:00

Mathematics, 13.12.2021 22:00



Social Studies, 13.12.2021 22:00

Biology, 13.12.2021 22:00

Biology, 13.12.2021 22:00

Mathematics, 13.12.2021 22:00

English, 13.12.2021 22:00







History, 13.12.2021 22:00



English, 13.12.2021 22:00