NH3(g) + Cl2(g) -> NH4Cl(s)

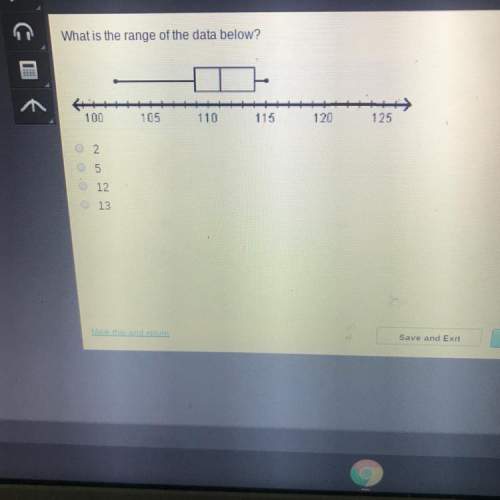
Mathematics, 18.04.2020 01:43 amison64
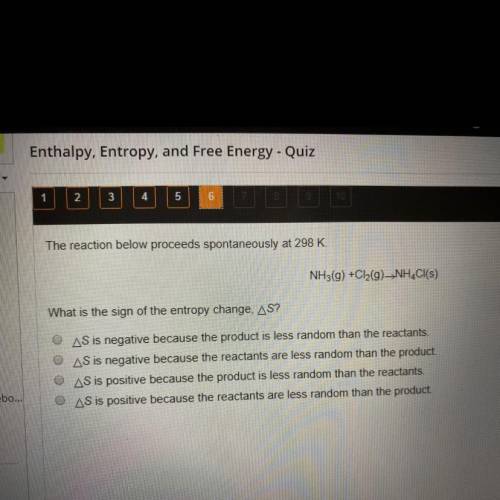
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
What is the sign of the entropy change, delta S?

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 17:00
(! ) three cylinders have a height of 8 cm. cylinder 1 has a radius of 1 cm. cylinder 2 has a radius of 2 cm. cylinder 3 has a radius of 3 cm. find the volume of each cylinder
Answers: 1

Mathematics, 21.06.2019 18:00
What is the measure of the smallest angle in the diagram? 15 29 32 45
Answers: 2


Mathematics, 22.06.2019 02:30
Quickly multiply and simplify polynomials (x – 4) (x^2 – 5x – 6)
Answers: 1
You know the right answer?
The reaction below proceeds spontaneously at 298 K.
NH3(g) + Cl2(g) -> NH4Cl(s)
NH3(g) + Cl2(g) -> NH4Cl(s)
Questions






Chemistry, 10.07.2019 16:30

Chemistry, 10.07.2019 16:30

Social Studies, 10.07.2019 16:30

Chemistry, 10.07.2019 16:30


Mathematics, 10.07.2019 16:30


Mathematics, 10.07.2019 16:30


Social Studies, 10.07.2019 16:30


Social Studies, 10.07.2019 16:30