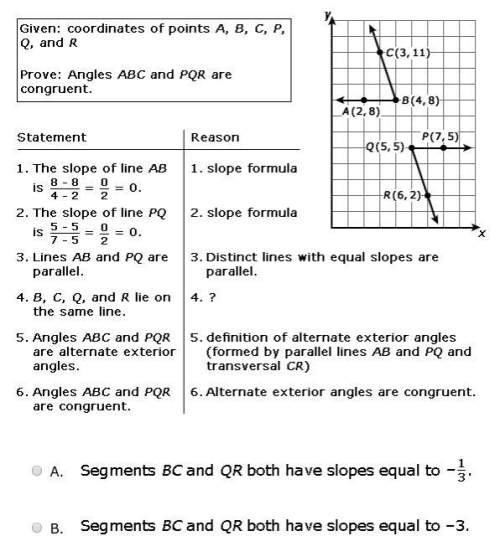
The reaction below is at dynamic equilibrium.
N2(g) + 3H2(g) +
2NH3(g)
...

Mathematics, 07.05.2020 04:05 kaayla20
The reaction below is at dynamic equilibrium.
N2(g) + 3H2(g) +
2NH3(g)
Which statement is true for the equilibrium system?
The concentration of NH, is greater than the concentration of N2.
The concentration of NH3 equals the concentration of N2.
The rate of the forward reaction equals the rate of the reverse reaction
The rate of the forward reaction is greater than the rate of the reverse reaction.

Answers: 2


Another question on Mathematics

Mathematics, 22.06.2019 00:10
Hello, i need compare km^2 and km. what's difference in this?
Answers: 2


Mathematics, 22.06.2019 01:20
Ahyperbola centered at the origin has a vertex at (-6,0) and a focus at (10,0)
Answers: 2

Mathematics, 22.06.2019 04:00
In a fruit survey, 300 children choose their favorite fruit out of apples, bananas, and watermelon. 150 chose apples and 90 chose bananas. what percent chose watermelon?
Answers: 1
You know the right answer?
Questions





Health, 28.06.2021 15:50

Spanish, 28.06.2021 15:50

Business, 28.06.2021 15:50



History, 28.06.2021 16:00


Physics, 28.06.2021 16:00

Advanced Placement (AP), 28.06.2021 16:00

Biology, 28.06.2021 16:00

Mathematics, 28.06.2021 16:00

Social Studies, 28.06.2021 16:00