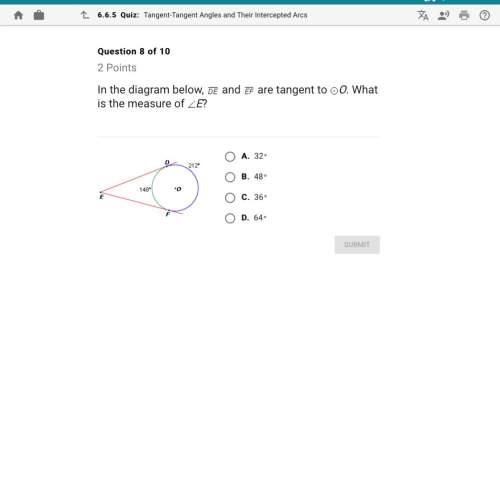
Mathematics, 27.06.2020 01:01 lizzyhearts
Please help
1. rubidium has two isotopes, 72.2% rubidium-85, and 27.8% rubidium-87. Calculate the average atomic mass of rubidium.
2. Bromine also has two isotopes: 50.7% is bromine-79 and 49.3% is bromine-81. Can you estimate the average atomic mass of bromine without using a calculator?
3. What is the average atomic mass of fluorine, whose only naturally occurring isotopes has an atomic mass of 19?

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 22:30
Complete the equation of the live through (-1,6) (7,-2)
Answers: 1

Mathematics, 22.06.2019 02:30
What are the triple angle formulas? is it related to double angle identities?
Answers: 1

Mathematics, 22.06.2019 04:00
If the equation of a line containing the midsegment of a triangle is y = -1/4x -11, what is the slope of the triangle side that is opposite the midsegment (the one not touching it). explain how you know.
Answers: 1

You know the right answer?
Please help
1. rubidium has two isotopes, 72.2% rubidium-85, and 27.8% rubidium-87. Calculate the a...
Questions

Mathematics, 06.05.2021 22:20


Social Studies, 06.05.2021 22:20

English, 06.05.2021 22:20



Chemistry, 06.05.2021 22:20


Mathematics, 06.05.2021 22:20



Chemistry, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20



Chemistry, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20

Mathematics, 06.05.2021 22:20