Mathematics, 07.07.2020 21:01 loganferg9202
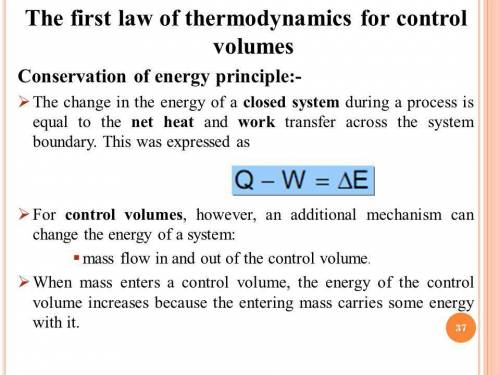
The first law of thermodynamics states that ΔE= Q− W. Is this also a statement of the principle of conservation of energy? No, the heat that is added to the system is only used to do work. No, the change in internal energy is the energy lost in the system. Yes, the heat added and the change in internal energy of the gas equal the work done by the piston. Yes, the heat that flows into the system is used to change the internal energy of the gas and becomes work done by the piston.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 19:20
The suare root of 9x plus 7 plus the square rot of 2x equall to 7
Answers: 1

Mathematics, 21.06.2019 19:30
At 6 1/2 cents per pound of string beans how much does 7 pounds cost
Answers: 1

Mathematics, 21.06.2019 20:00
Simplify (2^5/3^2)^4 a. 2^20/3^8 b. 2^9/3^8 c. 8^5/12^2 d. 2/3^2
Answers: 1

Mathematics, 21.06.2019 21:50
Match each whole number with a rational, exponential expression 3 square root 27^2find the domain and range of the exponential function h(x)=125^x. explain your findings. as x decreases does h increase or decrease? explain. as x increases does h increase or decrease? explain.
Answers: 3
You know the right answer?
The first law of thermodynamics states that ΔE= Q− W. Is this also a statement of the principle of c...
Questions



History, 09.09.2020 08:01


History, 09.09.2020 08:01

English, 09.09.2020 08:01


Mathematics, 09.09.2020 08:01

History, 09.09.2020 08:01


English, 09.09.2020 08:01

English, 09.09.2020 08:01


Mathematics, 09.09.2020 08:01



Mathematics, 09.09.2020 08:01

English, 09.09.2020 08:01

Social Studies, 09.09.2020 08:01