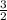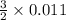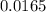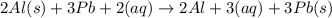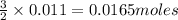Mathematics, 14.10.2019 11:00 carlo123
The equation for the reaction between al(s) and pb+2(aq) is:
2al(s) + 3pb+2(aq) 2al+2(aq) + 3pb(s)
therefore, for each 2 moles of al(s) that are used, how many moles of pb(s) are formed?
from the 0.011 mole of al(s) consumed, calculate the number of moles of pb(s) that should have been formed: mole(s)

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 18:00
If the cost to mail a package is an initial $5 and $0.28 for every pound the total cost to mail a package was $11.27, how much did the package weigh?
Answers: 1

Mathematics, 21.06.2019 20:50
Find the equation of a line that is perpendicular to line g that contains (p, q). coordinate plane with line g that passes through the points negative 3 comma 6 and 0 comma 5 3x − y = 3p − q 3x + y = q − 3p x − y = p − q x + y = q − p
Answers: 1

Mathematics, 22.06.2019 00:20
What is the slope of the line passing through the points (3, 3) and (5, 7) ? 1. 2 2. 1/2 3. −2 4. −1/2
Answers: 2

Mathematics, 22.06.2019 02:10
Which pair of expressions is equivalent? a 7(1–k)and7–k b 7(1–k)and1–7k c 7(1–k)and7–k d 7(1–k)and7–7k
Answers: 1
You know the right answer?
The equation for the reaction between al(s) and pb+2(aq) is:
2al(s) + 3pb+2(aq) 2al+2(a...
2al(s) + 3pb+2(aq) 2al+2(a...
Questions

Mathematics, 14.02.2020 03:49

Physics, 14.02.2020 03:49

Mathematics, 14.02.2020 03:49


History, 14.02.2020 03:49

Mathematics, 14.02.2020 03:49


Biology, 14.02.2020 03:49






Mathematics, 14.02.2020 03:50

Mathematics, 14.02.2020 03:50

Mathematics, 14.02.2020 03:50

English, 14.02.2020 03:50