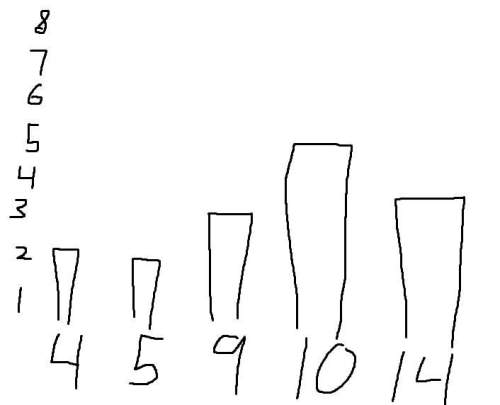Mathematics, 07.10.2020 21:01 ctyrector
The charged particles in the beams that Thomson studied came from atoms. As these particles moved away from their original atoms, they formed a visible beam. The current model of the atom includes protons, neutrons, and electrons. What is the best use of an atomic model to explain the charge of the particles in Thomson's beams? a. An atom's negative particles are surrounded by positive matter, so the positive particles are easier to remove. b. An atom's positive particles are surrounded by negative matter, so the negative particles are easier to remove. c. An atom's smaller negative particles are at a distance from the central positive particles, so the negative particles are easier to remove. d. An atom's larger positive particles are at a distance from the central negative particles, so the positive particles are easier to remove.

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 14:00
Which expression is equivalent to? assume x > 0 and y > 0.
Answers: 1


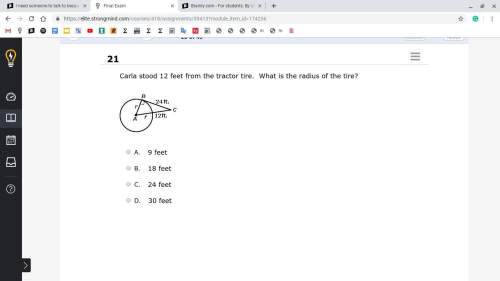
Mathematics, 21.06.2019 21:00
Glenda makes an online purchase for picture frames for $12.95 each and formats of $5.89 each the site says it taxes paid by the customer or 6.5% of the total purchase price shipping charges are based on the
Answers: 1

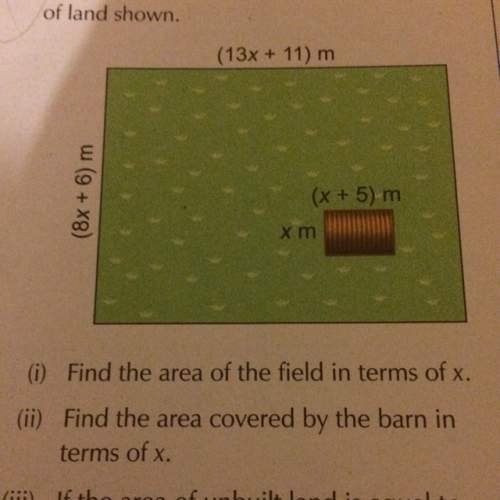
Mathematics, 21.06.2019 21:10
If f(x) and g(x) are inverse functions of each other, which of the following shows the graph of f(g(x)
Answers: 1
You know the right answer?
The charged particles in the beams that Thomson studied came from atoms. As these particles moved aw...
Questions

Mathematics, 03.07.2019 09:30

Mathematics, 03.07.2019 09:40

Chemistry, 03.07.2019 09:40

History, 03.07.2019 09:40

Mathematics, 03.07.2019 09:40



Mathematics, 03.07.2019 09:40


English, 03.07.2019 09:40

Mathematics, 03.07.2019 09:40


English, 03.07.2019 09:40

English, 03.07.2019 09:40

English, 03.07.2019 09:40

English, 03.07.2019 09:40

English, 03.07.2019 09:40

English, 03.07.2019 09:40

English, 03.07.2019 09:40