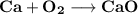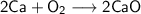Mathematics, 12.10.2020 18:01 Babybeast3544
When calcium metal (Ca) reacts with oxygen gas (O2), it produces the ionic compound calcium oxide (CaO). Write the balanced equation for this reaction. Then, explain if this process meets the requirements to be considered a redox reaction.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 16:30
The temperature of chicken soup is 192.7°f. as it cools, the temperature of the soup decreases 2.3°f per minute. what is the temperature in degrees fahrenheit of the soup after 25 minutes? a. 27.3°f b. 57.5°f c. 135.2°f d. 250.2°f
Answers: 1

Mathematics, 21.06.2019 17:40
The weight of full–grown tomatoes at a farm is modeled by a normal distribution with a standard deviation of 18.4 grams. the 95 percent confidence interval for the mean weight of the tomatoes is calculated using a sample of 100 tomatoes. what is the margin of error (half the width of the confidence interval)?
Answers: 2

Mathematics, 21.06.2019 22:30
If a line is defined by the equation y = 5x + 2, what is the slope?
Answers: 2

Mathematics, 22.06.2019 00:30
If you invest 1,500 today in a bank that gives you a 5 percent annual interest rate, which of these items can you buy in two years? a. electronics worth $1,650 b.fitness equipment worth $1,700 c.a holiday package worth $2,000
Answers: 2
You know the right answer?
When calcium metal (Ca) reacts with oxygen gas (O2), it produces the ionic compound calcium oxide (C...
Questions

Advanced Placement (AP), 06.05.2020 05:32


Health, 06.05.2020 05:32

Biology, 06.05.2020 05:32


Chemistry, 06.05.2020 05:32





English, 06.05.2020 05:32


Biology, 06.05.2020 05:32




Mathematics, 06.05.2020 05:32


Mathematics, 06.05.2020 05:32