Mathematics, 18.10.2020 07:01 Tyrant4life
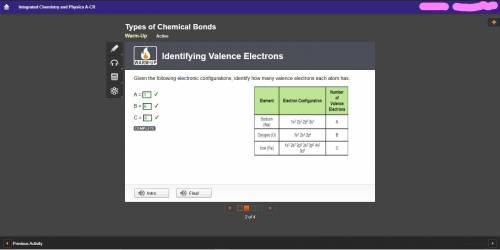
Given the following electronic configurations, identify how many valence electrons each atom has.
A =
B =
C =
A 3-column table with 3 rows. Column 1 is labeled Element with entries Sodium (upper N a), Oxygen (upper O), and Iron (upper F e). Column 2 is labeled electron configuration with entries 1 s superscript 2, 2 s superscript 2, 2 p superscript 6, 3 s superscript 1; 1 s super 2; 2 s super 2, 2 p super 4; and 1 s super 2, 2 s super 2, 2 p super 6, e p super 6, 4 s super 2, 3 d super 6. Column 3 is labeled number of valence electrons with entries A, B, and C.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 16:30
Which approach to the theory-and-research cycle does the following sequence reflect: (1) specific observations suggest generalizations, (2) generalizations produce a tentative theory, (3) the theory is tested through the formation of hypotheses, and (4) hypotheses may provide suggestions for additional observations?
Answers: 1


Mathematics, 22.06.2019 01:00
Why is causation so much more difficult to prove than correlation?
Answers: 2

Mathematics, 22.06.2019 03:00
Run a linear regression to determine an equation (y=mx+b y=mx+b)
Answers: 3
You know the right answer?
Given the following electronic configurations, identify how many valence electrons each atom has.
A...
Questions

Mathematics, 09.11.2020 17:20

Biology, 09.11.2020 17:20



Mathematics, 09.11.2020 17:20

Mathematics, 09.11.2020 17:20

Mathematics, 09.11.2020 17:20


Mathematics, 09.11.2020 17:20







Mathematics, 09.11.2020 17:20