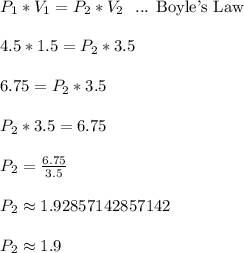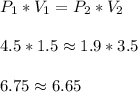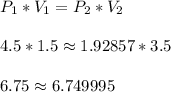Mathematics, 29.12.2020 20:30 ayoismeisalex
When a gas is kept at a constant temperature and pressure on it changes, its volume changes according to the following formula, known as Boyle’s law where P1 and V1 are the pressure (in atm) and the volume (in litres) at the beginning, and P2 and V2 are the pressure and the volume at the end. Find the final pressure P2 if V1 = 1.5 litres, P1 = 4.5 atm and V2 = 3.5 litres. Round to the nearest tenth of a atm.

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 23:10
You just purchased two coins at a price of $670 each. because one of the coins is more collectible, you believe that its value will increase at a rate of 7.1 percent per year, while you believe the second coin will only increase at 6.5 percent per year. if you are correct, how much more will the first coin be worth in 15 years?
Answers: 2

Mathematics, 21.06.2019 23:30
If you measured the width of a window in inches and then in feet with measurement would you have the greater number of units
Answers: 3

Mathematics, 21.06.2019 23:30
Simplify (8x2 − 1 + 2x3) − (7x3 − 3x2 + 1). −5x3 + 11x2 − 2 5x3 − 11x2 + 2 x3 + 2x2 + x3 x3 − 2x2 − x3
Answers: 1

You know the right answer?
When a gas is kept at a constant temperature and pressure on it changes, its volume changes accordin...
Questions

Mathematics, 11.05.2021 21:20





English, 11.05.2021 21:20

History, 11.05.2021 21:20

Spanish, 11.05.2021 21:20





Mathematics, 11.05.2021 21:20

Mathematics, 11.05.2021 21:20





Biology, 11.05.2021 21:20