Concentration of NO2 = 11.95

Mathematics, 08.01.2021 01:00 krystalhurst97
For the current reaction, 2NO2 ↔ N2O4, we have:
Kc = ![\frac{[N_{2} O_{4}]}{[NO_{2}]^{2} }](/tpl/images/2047/8468/2ef86.png)
Concentration of NO2 = 11.95
Concebtration of N2O4 = 6.05
Based on the current concentrations of NO2 and N2O4, what is Kc?
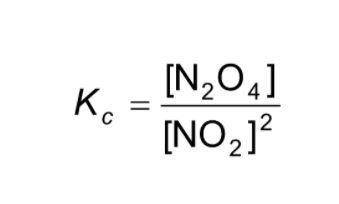

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 12:30
On the way home from visiting his family, vincent’s plane cruised at an altitude of 3.2 × 104 feet. he had heard that a man named felix baumgartner skydived from an altitude of 1.28 × 105 feet. vincent wants to know how much higher felix went on his skydiving trip. question 1 you have already seen how adding numbers works in either scientific notation or standard notation. but can you subtract numbers in scientific notation and get the same results as subtracting in standard notation? to find out, first solve vincent’s problem in standard notation. part a write the cruising altitude of vincent’s plane in standard notation.
Answers: 3


Mathematics, 21.06.2019 22:00
What is the length of the segment, endpoints of which are intersections of parabolas y=x^2− 11/4 x− 7/4 and y=− 7 /8 x^2+x+ 31/8 ?
Answers: 1

Mathematics, 22.06.2019 00:20
Does the construction demonstrate how to copy an angle correctly using technology a) yes the distance between points a and f was used to create circle h b) yes the distance between points f and g was used to create circle h c) no the distance between points a and f was used to create circle h d) no the distance between points f and g was used to create circle h
Answers: 3
You know the right answer?
For the current reaction, 2NO2 ↔ N2O4, we have:
Kc =
Concentration of NO2 = 11.95
Concentration of NO2 = 11.95
Questions


Biology, 04.11.2019 09:31

History, 04.11.2019 09:31

Social Studies, 04.11.2019 09:31

Social Studies, 04.11.2019 09:31

Mathematics, 04.11.2019 09:31

Biology, 04.11.2019 09:31

Mathematics, 04.11.2019 09:31


History, 04.11.2019 09:31



Computers and Technology, 04.11.2019 09:31





Computers and Technology, 04.11.2019 09:31

Biology, 04.11.2019 09:31

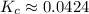
![\displaystyle K_c = \frac{[N_2O_4]}{[NO_2]^2}](/tpl/images/1020/5386/82efd.png)
![\displaystyle K_c = \frac{[6.05]}{[11.95]^2}](/tpl/images/1020/5386/714da.png) Exponents:
Exponents: ![\displaystyle K_c = \frac{[6.05]}{[142.803]}](/tpl/images/1020/5386/9a115.png) Divide:
Divide: 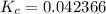 Round (Sig Figs):
Round (Sig Figs):