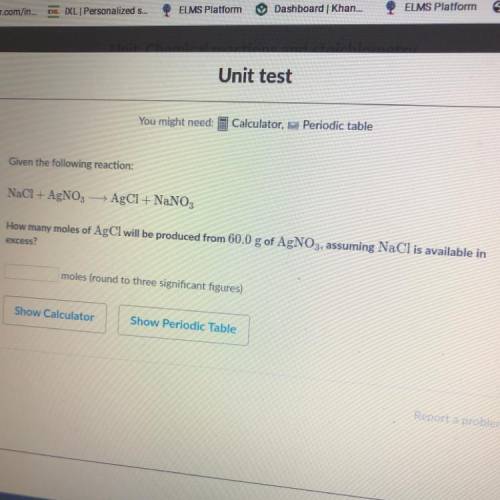
Mathematics, 17.02.2021 02:00 brifrog13
How many moles of AgCl will be produced from 60.0 g of AgNO3, assuming NaCl is available in excess?

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 16:30
Solve by any method (graphing, substitution or linear combination)y = x - 82x + 3y = 1a) (2, -6)b) (5, -3)c) (4, -4)d) (0, -8)i figured it out. the answer is (5, -3)
Answers: 1

Mathematics, 22.06.2019 01:30
Robert is placing sod in two square shaped areas of his backyard. one side of the first area is 7.5 feet. one side of the other area is 5.7 feet. the sod costs y dollars per square foot
Answers: 3

Mathematics, 22.06.2019 05:00
Will mark brainliest, , and, rate to the best answercompare the wins-to-losses ratios for the teams.
Answers: 2

Mathematics, 22.06.2019 05:00
Perry angelo is planning to open a restaurant. he has the resources to produce and sell either hamburgers or pizzas. so he does some research and finds that the new location has a good demand for pizza. he hires cooks, wait staff, and an accounts person for his new pizzeria. he also arranges for materials such as pizza ingredients and pizza boxes. determine three factors affecting perry's cost of making pizza. write down the name of the factors and their instances in this scenario.
Answers: 3
You know the right answer?
How many moles of AgCl will be produced from 60.0 g of AgNO3, assuming NaCl is available in
excess?...
Questions

Biology, 07.05.2020 00:58




Mathematics, 07.05.2020 00:58

Law, 07.05.2020 00:58

Mathematics, 07.05.2020 00:58

World Languages, 07.05.2020 00:58






History, 07.05.2020 00:59

Chemistry, 07.05.2020 00:59





Spanish, 07.05.2020 00:59