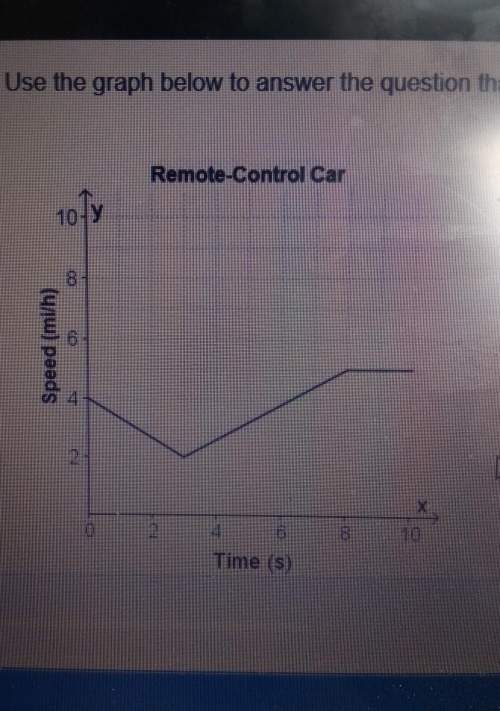
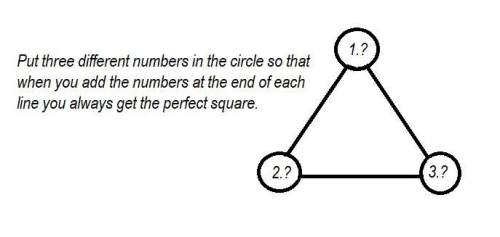
Mathematics, 03.04.2021 06:20 blake4033
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l): ΔH = −890 kJ Which statement about this reaction is correct? (3 points) Group of answer choices The reaction of one mole of oxygen (O2) absorbs 445 kJ of energy. The reaction of one mole of methane (CH4) absorbs 890 kJ of energy. The reaction of one mole of oxygen (O2) releases 445 kJ of energy. The reaction of two moles of methane (CH4) releases 890 kJ of energy.

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 17:00
Jamie went to home depot.she bought 25 bags of soil that cost $9 per bag.she bought 15 pots at $8 each, and she bought 23 bags of pebbles at $15 each.she used a coupon that gave her $5 off for every 100 dollars she spent. how much did jamie pay at the end?
Answers: 1

Mathematics, 21.06.2019 17:30
Terri makes a quilt using three sizes of fabric squares the side lenght of each fabric square is the square root of the area
Answers: 2

Mathematics, 21.06.2019 22:30
Aflagpole broke in a storm. it was originally 8 1 81 feet tall. 2 8 28 feet are still sticking straight out of the ground, where it snapped, but the remaining piece has hinged over and touches the ground some distance away. how far away is the end of the pole from the base of the pole along the ground?
Answers: 1

Mathematics, 21.06.2019 23:30
The complement of an angle is one-sixth the measure of the supplement of the angle. what is the measure of the complement angle?
Answers: 3
You know the right answer?
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l): ΔH = −890 kJ Which statement about this reaction is correct? (3...
Questions

Mathematics, 24.06.2019 20:00

Mathematics, 24.06.2019 20:00

Computers and Technology, 24.06.2019 20:00

Mathematics, 24.06.2019 20:00



English, 24.06.2019 20:00



Biology, 24.06.2019 20:00

History, 24.06.2019 20:00



Mathematics, 24.06.2019 20:00


Social Studies, 24.06.2019 20:00

Mathematics, 24.06.2019 20:00