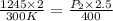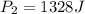Mathematics, 05.12.2019 10:31 laylah255
The pressure, p, of a gas varies directly with its temperature, t, and inversely with its volume, v, according to the equation p=nrt/v, where n is the number of molar units and r is the universal gas constant. one molar unit of gas has a pressure of about 1,245 joules at a temperature of 300 degrees kelvin and a volume of 2 liters. what is the pressure of the same number of molar units of the gas at a temperature of 400 degrees kelvin and a volume of 2.5 liters?

Answers: 3


Another question on Mathematics

Mathematics, 21.06.2019 13:20
Aboard game uses a fair six-sided die and a spinner with five equal-sized sections colored dark blue, green, light blue, red, and yellow. players roll the die and then spin the spinner. match each probability statement to its correct value.
Answers: 1

Mathematics, 22.06.2019 02:00
15 oranges weigh 3.75 kilograms (kg). if each orange weighs approximately the same, approximately how much does each orange weigh?
Answers: 2

Mathematics, 22.06.2019 02:30
Jennifer is 20 miles north of her house, and she is driving north on the highway at a rate of 55 miles per hour whats the slpoe
Answers: 2

Mathematics, 22.06.2019 03:00
25 ! what is the value of x? enter your answer in the box. x =
Answers: 2
You know the right answer?
The pressure, p, of a gas varies directly with its temperature, t, and inversely with its volume, v,...
Questions



Mathematics, 30.01.2021 05:30

Mathematics, 30.01.2021 05:30


Social Studies, 30.01.2021 05:30



Mathematics, 30.01.2021 05:30



Mathematics, 30.01.2021 05:30


Biology, 30.01.2021 05:30



English, 30.01.2021 05:30



Mathematics, 30.01.2021 05:30

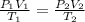 (for same value of n)
(for same value of n)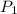 = initial pressure of gas = 1245 J
= initial pressure of gas = 1245 J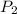 = final pressure of gas = ?
= final pressure of gas = ?
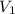 = initial volume of gas = 2 L
= initial volume of gas = 2 L
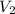 = final volume of gas = 2.5L
= final volume of gas = 2.5L
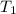 = initial temperature of gas = 300K
= initial temperature of gas = 300K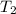 = final temperature of gas = 400K
= final temperature of gas = 400K