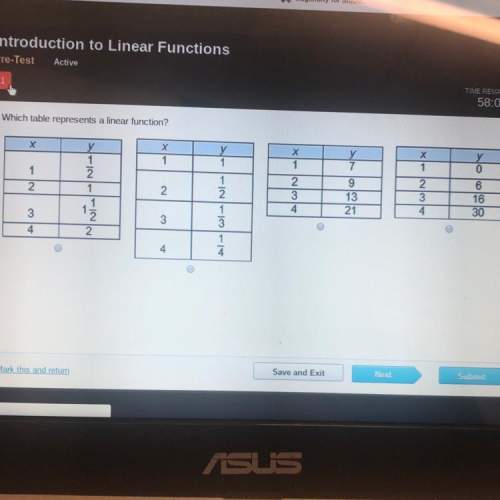
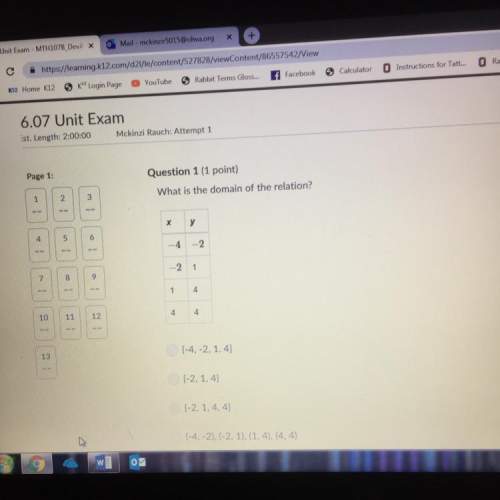
Mathematics, 07.06.2021 23:50 gemouljr
If 12.5 grams of calcium is reacted with excess hydrogen chloride in a water solution
according to the following chemical reaction, and the measured amount of 23.5
grams of calcium chloride is produced, what is the percent
yield for the reaction? Use
the balanced equation given below.
Ca (s) + 2HCl (aq) - CaCl2 (aq) + H2 (g)
First, calculate the theoretical yield, then calculate the percent yield. You must show
all your work with calculations with a proper set up including:
a. how you find the molar mass of CaCl2,
b. how you calculate the theoretical yield
C. how you calculate the correct percent yield.

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 20:00
You wanted to draw an enlargement of design that printed on a card that is 4 in by 5
Answers: 1


Mathematics, 22.06.2019 00:30
Officer brimberry wrote 32 tickets for traffic violations last week, but only 4 tickets this week. what is the percent decrease? give your answer to the nearest tenth of a percent.
Answers: 1

Mathematics, 22.06.2019 01:00
Which angle has the same measure as the dehedral angle formed by the orange face and the purple rectangle
Answers: 1
You know the right answer?
If 12.5 grams of calcium is reacted with excess hydrogen chloride in a water solution
according to...
Questions

Mathematics, 16.01.2021 18:40

Mathematics, 16.01.2021 18:40


English, 16.01.2021 18:40

Health, 16.01.2021 18:40

Mathematics, 16.01.2021 18:40



English, 16.01.2021 18:40



English, 16.01.2021 18:40

Mathematics, 16.01.2021 18:40


Mathematics, 16.01.2021 18:40

Mathematics, 16.01.2021 18:40

English, 16.01.2021 18:40

Mathematics, 16.01.2021 18:50

Mathematics, 16.01.2021 18:50

Mathematics, 16.01.2021 18:50