Mathematics, 29.06.2021 20:20 hokamidat
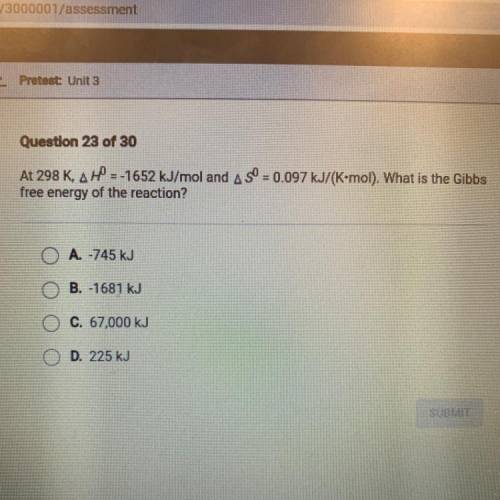
At 298 K, AH = -1652 kJ/mol and AS = 0.097 kJ/(K-mol). What is the Gibbs free energy of the reaction?

Answers: 1


Another question on Mathematics

Mathematics, 21.06.2019 14:30
Fiona and her friends are playing a game by guessing where a coin will land when it is randomly dropped inside the square shown below. fiona guesses that the coin is likely to land in the blue area. which explains whether or not fiona is correct and why?
Answers: 1

Mathematics, 21.06.2019 17:30
Danielle earns a 7.25% commission on everything she sells at the electronics store where she works. she also earns a base salary of $750 per week. what were her sales last week if her total earnings for the week were $1,076.25?
Answers: 3

Mathematics, 21.06.2019 22:00
What is the value of the discriminant of the quadratic equation -2x = -8x + 8 and what does its value mean about thenumber of real number solutions the equation has?
Answers: 3

You know the right answer?
At 298 K, AH = -1652 kJ/mol and AS = 0.097 kJ/(K-mol). What is the Gibbs
free energy of the reactio...
Questions

English, 18.12.2020 04:10

Chemistry, 18.12.2020 04:10

German, 18.12.2020 04:20

Mathematics, 18.12.2020 04:20

Mathematics, 18.12.2020 04:20

History, 18.12.2020 04:20

History, 18.12.2020 04:20




Advanced Placement (AP), 18.12.2020 04:20



English, 18.12.2020 04:20




Biology, 18.12.2020 04:20


Mathematics, 18.12.2020 04:20