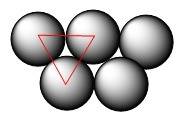
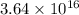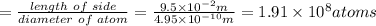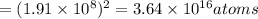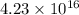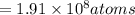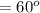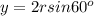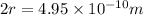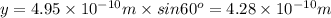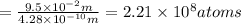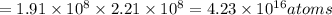Using arrangement (a), how many rb atoms could be placed on a square surface that is 9.5 cm on a side? the diameter of a rubidium atom is 4.95å. we will consider two different ways of placing the atoms on a surface. in arrangement (a), all the atoms are lined up with one another. arrangement (b) is called a close-packed arrangement because the atoms sit in the "depressions" formed by the previous row of atoms:

Answers: 1


Another question on Physics

Physics, 22.06.2019 16:50
Two loudspeakers, 5.5 m apart and facing each other, play identical sounds of the same frequency. you stand halfway between them, where there is a maximum of sound intensity. moving from this point toward one of the speakers, you encounter a minimum of sound intensity when you have moved 0.33 m . assume the speed of sound is 340 m/s.part a) what is the frequency of the sound? part b) if the frequency is then increased while you remain 0.21 m from the center, what is the first frequency for which that location will be a maximum of sound intensity? express your answer to two significant figures and include the appropriate units.
Answers: 2

Physics, 22.06.2019 21:20
Abeverage can is made of 3004-h19 aluminum alloy (elastic modulus 69 gpa, tensile yield strength 285 mpa, density 2.72 g/cm^3). the dimensions on the can are approximated as a thin-walled cylinder with a height of 4.83 inches, diameter of 2.60 inches. empty the can has a mass of 14.2 g. determine: a. the wall thickness of the cylinder b. assuming a pinned-pinned condition what is the critical load? c. assuming a fixed-fixed condition what is the critical load?
Answers: 1

Physics, 23.06.2019 01:30
Which statements did both aristotle and ptolemy assume? check all that apply.
Answers: 1

You know the right answer?
Using arrangement (a), how many rb atoms could be placed on a square surface that is 9.5 cm on a sid...
Questions



Health, 09.12.2019 09:31



SAT, 09.12.2019 09:31



Mathematics, 09.12.2019 09:31

History, 09.12.2019 09:31

Health, 09.12.2019 09:31

Spanish, 09.12.2019 09:31

History, 09.12.2019 09:31

Mathematics, 09.12.2019 09:31