Physics, 11.07.2019 14:00 andrejr0330jr
In a polar nitrogen trifluoride, nf3 molecule, nitrogen and fluorine atoms share electrons. the fluorine atoms have a stronger attraction for electrons than the nitrogen atom has. what can be determined from the molecule's polarity? a. it has polar bonds and is symmetric. b. it has ionic bonds and is symmetric. c. it has polar bonds and is asymmetric. d. it has nonpolar bonds and is asymmetric.

Answers: 1


Another question on Physics

Physics, 22.06.2019 09:00
Chemical energy is a form of energy. a. heat b. kinetic c. potential d. electromagnetic
Answers: 2

Physics, 22.06.2019 09:30
An electric clothes dryer has a resistance of 8 ohms. it draws 30 a of current. what is the voltage, in volts, of the wall outlet that it is plugged into?
Answers: 2

Physics, 22.06.2019 13:00
Nacidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.
Answers: 1

Physics, 22.06.2019 17:30
Convection currents are caused by differences in what two things?
Answers: 1
You know the right answer?
In a polar nitrogen trifluoride, nf3 molecule, nitrogen and fluorine atoms share electrons. the fluo...
Questions


Mathematics, 20.07.2019 11:00

Biology, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00




Mathematics, 20.07.2019 11:00

Mathematics, 20.07.2019 11:00


Mathematics, 20.07.2019 11:00



Mathematics, 20.07.2019 11:00



Mathematics, 20.07.2019 11:00


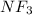 molecule, nitrogen has 5 valence electrons and F has 7 valence electron, 3 electrons of nitrogen are shared with 3 Fluorine atoms and 2 electrons are left on nitrogen atom as lone-pair.
molecule, nitrogen has 5 valence electrons and F has 7 valence electron, 3 electrons of nitrogen are shared with 3 Fluorine atoms and 2 electrons are left on nitrogen atom as lone-pair.