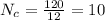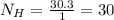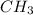Answers: 1


Another question on Physics

Physics, 21.06.2019 18:00
Ineed extreme for task 2. my student number is 571721. many to whoever solves this correctly, im in debt to you.
Answers: 2


Physics, 21.06.2019 21:50
What is the length x of the side of the triangle below? (hint: use the cosine function)
Answers: 1

Physics, 22.06.2019 08:50
You are a sales representative for a company that makes a new alternate fuel for vehicles. you have prepared a presentation for the environmental engineers to sell your new product. what question do you expect the audience to ask regarding whether the new fuel will cause less damage to the environment? a. do we have to change any parts of the vehicle to use this alternate fuel? b. will the vehicles get better fuel mileage with the alternate fuel? c. how much greenhouse gas does your fuel produce compared with current fuel sources? d. is the alternate fuel more expensive than fossil fuel?
Answers: 2
You know the right answer?
One mole of an unknown sample contains 120 g of carbon and 30.3 g of hydrogen. what is the empirical...
Questions

Health, 05.10.2019 07:30

Social Studies, 05.10.2019 07:30

English, 05.10.2019 07:30

Mathematics, 05.10.2019 07:30

Mathematics, 05.10.2019 07:30

Chemistry, 05.10.2019 07:30

Physics, 05.10.2019 07:30


Biology, 05.10.2019 07:30


Mathematics, 05.10.2019 07:30

Computers and Technology, 05.10.2019 07:30

History, 05.10.2019 07:30






Mathematics, 05.10.2019 07:30