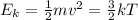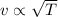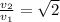Physics, 04.02.2020 14:54 smiley29162
Which of the following statements is not a correct assumption of the classicalmodel of an ideal gas? a. the molecules are in random motion. b. the volume of the molecules is negligible compared with the volume occupied bythe gas. c. the molecules obey newton's laws of motion. d. the collisions between molecules are inelastic. e. the only appreciable forces on the molecules are those that occur duringcollisions. a sample of an ideal gas is in a tank of constant volume. the sample absorbsheat energy so that its temperature changes from 300 k to 600 k. if v1 is theaverage speed of the gas molecules before the absorption of heat and v2 is theiraverage speed after the absorption of heat, what is the ratio v2/ v1? a. 1/2 b. 1 c. 2 d. 2 e. 4

Answers: 3


Another question on Physics

Physics, 22.06.2019 00:30
Which is not one of the major climate zones? question 3 options: rain forest polar tropical temperate
Answers: 1

Physics, 22.06.2019 03:30
Two polarizers are oriented at 24.0∘ to one another. light polarized at a 12.0-degree angle to each polarizer passes through both. what is the transmitted intensity (%)?
Answers: 2

Physics, 22.06.2019 08:00
What is a carrier wave and how does it affect what you hear on the radio
Answers: 1

Physics, 22.06.2019 13:20
It is reasonable to assume that the bulk modulus of blood is about the same as that of water (2.2 gpa). as one goes deeper and deeper in the ocean, the pressure increases by 10000 pa for every meter below the surface. if a diver goes down 80.0 m in the ocean, by how much does each cubic centimeter of her blood change in volume? give the answer in cubic centimeters (actually one cubic centimeter equals one milliliter).
Answers: 2
You know the right answer?
Which of the following statements is not a correct assumption of the classicalmodel of an ideal gas?...
Questions


Physics, 25.09.2020 08:01

Mathematics, 25.09.2020 08:01




English, 25.09.2020 08:01



English, 25.09.2020 08:01





History, 25.09.2020 08:01

Mathematics, 25.09.2020 08:01


Chemistry, 25.09.2020 08:01


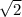
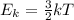
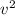 :
: