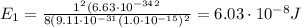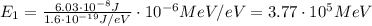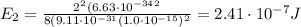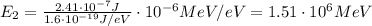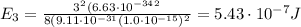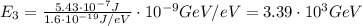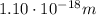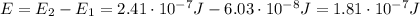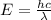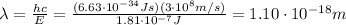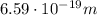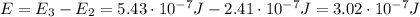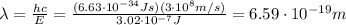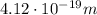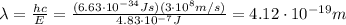(a) find the energy of the ground state (n = 1) and the first two excited states of an electron in a one-dimensional box of length l = 1.0 10-15 m = 1.00 fm (about the diameter of an atomic nucleus). ground state mev first excited state mev second excited state gev make an energy-level diagram for the system. (do this on paper. your instructor may ask you to turn in this work.) (b) calculate the wavelength of electromagnetic radiation emitted when the electron makes a transition from n = 2 to n = 1. fm (c) calculate the wavelength of electromagnetic radiation emitted when the electron makes a transition from n = 3 to n = 2. fm (d) calculate the wavelength of electromagnetic radiation emitted when the electron makes a transition from n = 3 to n = 1. fm

Answers: 2


Another question on Physics

Physics, 21.06.2019 14:50
Ingrid wrote the hypothesis below. if the temperature of a liquid increases, the density of the liquid decreases because the particles move farther apart. what are the variables in her hypothesis?
Answers: 3

Physics, 21.06.2019 15:10
Consider the following problem: a box with an open top is to be constructed from a square piece of cardboard, 3 ft wide, by cutting out a square from each of the four corners and bending up the sides. find the largest volume that such a box can have.
Answers: 3

Physics, 21.06.2019 22:30
The percent efficiency of a machine can never be 100% (or greater), because in the real world some energy is always converted into a. heat b. work c. input force d. output force
Answers: 1

Physics, 22.06.2019 06:00
What will a positive and a negative charge do if they are separated from each other?
Answers: 3
You know the right answer?
(a) find the energy of the ground state (n = 1) and the first two excited states of an electron in a...
Questions

English, 05.12.2020 19:20



Computers and Technology, 05.12.2020 19:20

Mathematics, 05.12.2020 19:20

Mathematics, 05.12.2020 19:20

World Languages, 05.12.2020 19:20






Mathematics, 05.12.2020 19:20

History, 05.12.2020 19:20

Mathematics, 05.12.2020 19:20

Mathematics, 05.12.2020 19:20

Mathematics, 05.12.2020 19:20

Physics, 05.12.2020 19:20


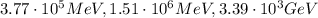
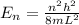
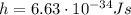 is the Planck constant
is the Planck constant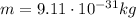 is the mass of the electron
is the mass of the electron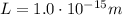 is the size of the box
is the size of the box