Physics, 24.06.2019 10:20 saucyboyFredo
Given 4.8 moles of a gas at 37 degrees celsius and at 792 torr, what is the volume of the gas? (the ideal gas constant is 0.0821 l · atm/mol · k and 1 atm = 760 torr.) 1.40 x 101 l 1.84 x 10-2 l 1.17 x 102 l 1.54 x 10-1 l

Answers: 2


Another question on Physics


Physics, 22.06.2019 06:20
Atwo-stage air compressor operates at steady state, compression 10m^3/min of air from 100 kpa and 300k to 1200 kpa. an intercooler between the two stages cools the air to 300k at a constant pressure of 350 kpa. the compression processes are isentropic. a) calculate the power required to run the compressor, in kw b) compare the result to the power required for isentropic compression from the same inlet state to the same final pressure.
Answers: 1

Physics, 22.06.2019 15:00
A10 kg box initially at rest is pulled 18 meters across a horizontal, frictionless surface by a 40 n force. what is the block’s final velocity over the 18 meters?
Answers: 2

Physics, 22.06.2019 17:30
This chart shows characteristics of three different types of waves. which statement is best supported by the information in the chart? wave x and wave y are mechanical waves, and wave z is an electromagnetic wave. wave x and wave y are electromagnetic waves, and wave z is a mechanical wave. wave x and wave z are electromagnetic waves, and wave y is a mechanical wave. wave x and wave z are mechanical waves, and wave y is an electromagnetic wave.
Answers: 1
You know the right answer?
Given 4.8 moles of a gas at 37 degrees celsius and at 792 torr, what is the volume of the gas? (the...
Questions


Mathematics, 07.10.2021 02:20




English, 07.10.2021 02:20


Engineering, 07.10.2021 02:30

Mathematics, 07.10.2021 02:30




Computers and Technology, 07.10.2021 02:30

English, 07.10.2021 02:30

Computers and Technology, 07.10.2021 02:30





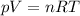
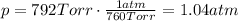 is the pressure
is the pressure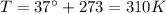 is the temperature
is the temperature