
Physics, 03.07.2019 22:10 Carrchris021
The temperature of a pot of water is 90.0 °c and the mass of the water is 112 g. a block with a mass of 21.0 g and a temperature of 20.0°c is dropped into the water. the final temperature of the water and the block is 88.3 °c. the specific heat of water is exactly 1 cal/g.°c. how much heat was lost by the water? o 1.90 x10^2 cal o 35.7 cal o 7650 cal o 1.70 cal how much heat was gained by the block? o 1430 cal o 190 x10^2 cal o 35.7 cal o 68.3 cal what is the specific heat of the block? o 0.133 cal/g.°c o 5.33 cal/g.°c o273000 cal/g.°c o0.0249 cal/g.°c

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:30
Acar moves at 12m/s and coasts up a hill with a uniform acceleration of -1.6m/s2. how far has it traveled after 6.0s?
Answers: 1

Physics, 22.06.2019 03:30
What makes thermal imaging cameras useful? they can detect differences in color. they can detect differences in wave speeds. they can detect differences in temperature. they can detect mechanical waves.
Answers: 1

Physics, 22.06.2019 10:00
During which interval are the persons represented by the graph not moving? a) 0 to 0.5 hrs b) 1.5 to 2 hrs c) 2 to 2.5 hrs d) 4.5 to 5 hrs
Answers: 1

Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1
You know the right answer?
The temperature of a pot of water is 90.0 °c and the mass of the water is 112 g. a block with a mass...
Questions



Social Studies, 30.09.2019 06:00

Mathematics, 30.09.2019 06:00





Health, 30.09.2019 06:00

Social Studies, 30.09.2019 06:00

Social Studies, 30.09.2019 06:00

Mathematics, 30.09.2019 06:00


Health, 30.09.2019 06:00



History, 30.09.2019 06:00


Business, 30.09.2019 06:00

Social Studies, 30.09.2019 06:00

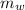 = mass of water = 112 g
= mass of water = 112 g 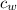 = specific heat of water = 1 cal/(g °C)
= specific heat of water = 1 cal/(g °C)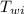 = initial temperature of water = 90.0 °C
= initial temperature of water = 90.0 °C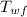 = final temperature of water = 88.3 °C
= final temperature of water = 88.3 °C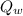 = Heat lost by water
= Heat lost by water 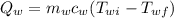
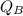 = Heat gained by the block
= Heat gained by the block 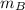 = mass of block = 21.0 g
= mass of block = 21.0 g 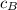 = specific heat of block
= specific heat of block 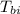 = initial temperature of block = 20.0 °C
= initial temperature of block = 20.0 °C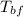 = final temperature of block = 88.3 °C
= final temperature of block = 88.3 °C

