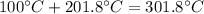Answers: 1


Another question on Physics

Physics, 21.06.2019 20:00
The sun generates both mechanical and electromagnetic waves. which statement about those wave is true?
Answers: 1

Physics, 22.06.2019 16:50
Acommercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at -35°c by rejecting waste heat to cooling water that enters the condenser at 18°c at a rate of 0.25 kg/s and leaves at 26°c. the refrigerant enters the condenser at 1.2 mpa and 50°c. if the compressor consumes 3.3 kw of power, determine (a) the mass flow rate of the refrigerant, (b) the refrigeration load, (c) the cop, and (d) the minimum power input to the compressor for the same refrigeration load.
Answers: 2

Physics, 22.06.2019 17:00
Adiver named jacques observes a bubble of air rising from the bottom of a lake (where the absolute pressure is 3.50 atm) to the surface (where the pressure is 1.00 atm). the temperature at the bottom is 4.00 ∘c, and the temperature at the surface is 23.0 ∘c.what is the ratio of the volume of the bubble as it reaches the surface (vs) to its volume at the bottom (vb)? if jaques were to hold his breath the air in his lungs would be kept at a constant temperature. would it be safe for jacques to hold his breath while ascending from the bottom of the lake to the surface?
Answers: 1

Physics, 22.06.2019 23:00
Which type of reaction is shown in this energy diagram? energy products activation energy reactants time o a. endothermic, because energy is released to the surroundings o b. exothermic, because energy is absorbed from the surroundings o c. exothermic, because energy is released to the surroundings o d. endothermic, because energy is absorbed from the surroundings
Answers: 1
You know the right answer?
19. determine the final state and its temperature when 150.0 kj of heat are added to 50.0 g of water...
Questions

Business, 25.01.2021 21:00



Mathematics, 25.01.2021 21:00

Health, 25.01.2021 21:00




Health, 25.01.2021 21:00

Mathematics, 25.01.2021 21:00

Mathematics, 25.01.2021 21:00

Chemistry, 25.01.2021 21:00


Mathematics, 25.01.2021 21:00


History, 25.01.2021 21:00

Computers and Technology, 25.01.2021 21:00

Physics, 25.01.2021 21:00

Physics, 25.01.2021 21:00

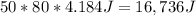 . To pass the boiling point,
. To pass the boiling point, 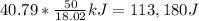 are necessary (18.02 is the molar mass of water). This means that
are necessary (18.02 is the molar mass of water). This means that 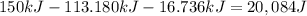 are left. This allows the steam to heat another
are left. This allows the steam to heat another 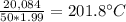 . Therefore, it ends as steam at temperature
. Therefore, it ends as steam at temperature