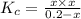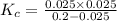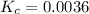Physics, 18.07.2019 21:20 einstein101
If 0.40 moles of pcl5 is heated in a 10.0 l container, equilibrium is established in which 0.25 moles of cl2 are present. the reaction is: pcl5(g)\longleftrightarrow ⟺ pcl3(g) + cl2(g) what is the value of the equilibrium constant?

Answers: 1


Another question on Physics


Physics, 22.06.2019 21:30
What describes the formation of horizon b? a. forms at the surface b. features parent material c. undergoes the most change d. forms due to decomposed material i think the answer is c. undergoes the most change
Answers: 1

Physics, 23.06.2019 06:50
Un conductor circula por una carretera en una velocidad de 90 km/h y b que se enciende la luz amarilla de un semáforo situado una distancia de 150 m. si el semáforo tarda tres segundos en cambiar a rojo el coche frena con una aceleración de 2 m por segundos 2¿ cometer una infracción ese conductor?
Answers: 1

Physics, 23.06.2019 10:00
Work requires a force which causes: potential energy or motion
Answers: 2
You know the right answer?
If 0.40 moles of pcl5 is heated in a 10.0 l container, equilibrium is established in which 0.25 mole...
Questions

Biology, 17.04.2020 06:21





Mathematics, 17.04.2020 06:26


Mathematics, 17.04.2020 06:26

Law, 17.04.2020 06:26

Mathematics, 17.04.2020 06:26

Mathematics, 17.04.2020 06:26



English, 17.04.2020 06:27

Mathematics, 17.04.2020 06:27


Mathematics, 17.04.2020 06:27


Arts, 17.04.2020 06:27

Mathematics, 17.04.2020 06:27

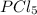 = 2 mole
= 2 mole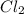 at equilibrium= 0.25 mole
at equilibrium= 0.25 mole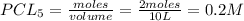
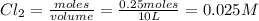
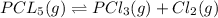
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/0105/4048/ffe89.png)