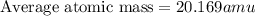Answers: 2


Another question on Physics

Physics, 22.06.2019 14:40
14. a body is projected with velocity vi from a.at the same time another body is projectedvertically upwards from point b withvelocity v2 lies vertically below the highestpoint. both the bodies collide thenv2÷v1is
Answers: 1


Physics, 22.06.2019 16:50
Acommercial refrigerator with refrigerant-134a as the working fluid is used to keep the refrigerated space at -35°c by rejecting waste heat to cooling water that enters the condenser at 18°c at a rate of 0.25 kg/s and leaves at 26°c. the refrigerant enters the condenser at 1.2 mpa and 50°c. if the compressor consumes 3.3 kw of power, determine (a) the mass flow rate of the refrigerant, (b) the refrigeration load, (c) the cop, and (d) the minimum power input to the compressor for the same refrigeration load.
Answers: 2

Physics, 22.06.2019 20:20
The base of a 50-meter tower is at the origin; the base of a 50-meter tree is at (0, 50, 0). the ground is flat and the z-axis points upward. the following parametric equations describe the motion of six projectiles each launched at time t = 0 in seconds. (i) r (t) = (50 + t2)k (ii) r (t) = 2t2 j + 2t2k (iii) r (t) = 50 i + 50 j + (50 − t2)k (iv) r (t) = 2t j + (50 − t2)k (v) r (t) = (50 − 2t) i + 2t j + (50 − t)k (vi) r (t) = t i + t j + tk (a) which projectile is launched from the top of the tower and goes downward? at time t = , the projectile hits the ground at point (x, y, z) = . (b) which projectile hits the top of the tree?
Answers: 2
You know the right answer?
An element has the following natural abundances and isotopic masses: 90.92% abundance with 19.99 am...
Questions

Computers and Technology, 19.07.2019 05:30

Mathematics, 19.07.2019 05:30

Computers and Technology, 19.07.2019 05:30




Social Studies, 19.07.2019 05:30

Business, 19.07.2019 05:30


Biology, 19.07.2019 05:30


Health, 19.07.2019 05:30



Computers and Technology, 19.07.2019 05:30

Computers and Technology, 19.07.2019 05:30




Mathematics, 19.07.2019 05:30

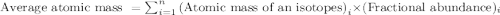 .....(1)
.....(1)![\text{Average atomic mass}=[(19.99\times 0.9092)+(20.99\times 0.0026)+(21.99\times 0.0882)]](/tpl/images/0105/8735/94c3c.png)