Answers: 2


Another question on Physics


Physics, 23.06.2019 02:00
How does changing the frequency affect the energy of the wave?
Answers: 3

Physics, 23.06.2019 03:00
Ablock of mass m slides on a horizontal frictionless table with an initial speed v0 . it then compresses a spring of force constant k and is brought to rest. the acceleration of gravity is 9.8 m/s2 . how much is the spring compressed x from its natural length?
Answers: 3

Physics, 23.06.2019 03:30
Aspring that is stretched 2.6 cm stores a potential energy of 0.053 j. what is the spring constant of this spring?
Answers: 1
You know the right answer?
The frequency factor and activation energy for a chemical reaction are a = 4.23 x 10–12 cm3/(molecul...
Questions

Mathematics, 29.04.2021 19:50

Health, 29.04.2021 19:50





Chemistry, 29.04.2021 19:50

Mathematics, 29.04.2021 19:50


Arts, 29.04.2021 19:50

Mathematics, 29.04.2021 19:50

Biology, 29.04.2021 19:50

Mathematics, 29.04.2021 19:50


Social Studies, 29.04.2021 19:50


Mathematics, 29.04.2021 19:50



Mathematics, 29.04.2021 19:50

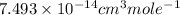
![k=A\times e^{[\frac{-Ea}{RT}]}](/tpl/images/0105/9531/eef14.png)
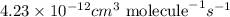
![k=4.23\times 10^{-12}cm^3\text{ molecule}^{-1}s^{-1}\times e^{[\frac{-12.9kJ/mol}{(8.314J/mole.K)\times (384.7K)}]}](/tpl/images/0105/9531/82c0d.png)