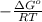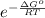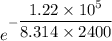Physics, 19.07.2019 04:20 javontiye226
When adjusted for any changes in δh and δs with temperature, the standard free energy change δg∘t at 2400 k is equal to 1.22×105j/mol . calculate the equilibrium constant at 2400 k .

Answers: 2


Another question on Physics

Physics, 21.06.2019 20:10
Which force is most responsible for binding together an atom's protons and neutrons? electrostatic gravitational nuclear magnetic
Answers: 1

Physics, 22.06.2019 10:30
Carbon is allowed to diffuse through a steel plate 15 mm thick. the concentrations of carbon at the two faces are 0.65 and 0.30 kg c/m^3 fe, which are maintained constant. if the preexponential and activation energy are 6.2 x 10-7 m2 /s and 80,000 j/mol, respectively, compute the temperature at which the diffusion flux is 1.43 x 10^-9 kg/m^2 -s.
Answers: 3


Physics, 23.06.2019 01:40
When you see distant streetlights through smog, they look dimmer and redder than they do normally. but when you see the same streetlights through fog or falling snow, they look dimmer but not redder. use your knowledge of the interstellar medium to discuss the relative sizes of the particles in smog, fog, and snowstorms compared to the wavelength of light.
Answers: 3
You know the right answer?
When adjusted for any changes in δh and δs with temperature, the standard free energy change δg∘t at...
Questions

Mathematics, 30.04.2021 22:10


World Languages, 30.04.2021 22:10




Mathematics, 30.04.2021 22:10


Mathematics, 30.04.2021 22:10



Chemistry, 30.04.2021 22:10

Business, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10

Mathematics, 30.04.2021 22:10


Mathematics, 30.04.2021 22:10

Computers and Technology, 30.04.2021 22:10


Computers and Technology, 30.04.2021 22:10