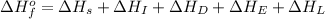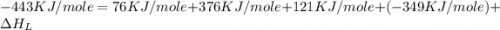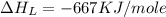Physics, 20.07.2019 04:10 gunaranjan09
The overall energy involved in the formation of cscl from cs(s) and cl2(g) is −443 kj/mol. given the following information: heat of sublimation for cs is +76 kj/mol, bond dissociation energy for 12cl2 is +121 kj/mol, ei1 for cs is +376 kj/mol, and eea for cl(g) is −349 kj/mol. what is the magnitude of the lattice energy for cscl?

Answers: 2


Another question on Physics

Physics, 22.06.2019 02:30
Acar moves at 12m/s and coasts up a hill with a uniform acceleration of -1.6m/s2. how far has it traveled after 6.0s?
Answers: 1

Physics, 22.06.2019 08:00
Based on the concept of the wave-like nature of light, huygens' theory of light postulates that the more light was "bent" by a substance the slower it would move while traversing across that substance. a) deflection b) interference c) refraction d) resonance
Answers: 3

Physics, 22.06.2019 14:30
What is the relationship between the direction of motion of the balloon and the wind currents
Answers: 1

Physics, 22.06.2019 16:00
Suppose a soccer ball is kicked from the ground at an angle 20.0º above the horizontal at 8.00 m/s. the y-velocity is determined to be 2.74 m/s. how long will the ball be in the air? assume the ball lands at the same height at which it was kicked.
Answers: 2
You know the right answer?
The overall energy involved in the formation of cscl from cs(s) and cl2(g) is −443 kj/mol. given the...
Questions


History, 29.01.2020 12:43



Mathematics, 29.01.2020 12:43


History, 29.01.2020 12:43




Mathematics, 29.01.2020 12:43



Biology, 29.01.2020 12:43



English, 29.01.2020 12:43



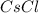 :
: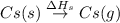
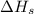 = sublimation energy of calcium
= sublimation energy of calcium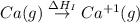
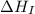 = ionization energy of calcium
= ionization energy of calcium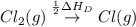
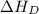 = dissociation energy of chlorine
= dissociation energy of chlorine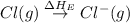
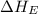 = electron affinity energy of chlorine
= electron affinity energy of chlorine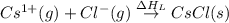
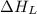 = lattice energy of calcium chloride
= lattice energy of calcium chloride