
A31.15−g stainless steel ball bearing at 103.27°c is placed in a constant-pressure calorimeter containing 110.3 g of water at 22.59°c. if the specific heat of the ball bearing is 0.474 j / (g · °c), calculate the final temperature of both the water and steel when they equilibrate. assume the calorimeter to have negligible heat capacity.

Answers: 1


Another question on Physics

Physics, 21.06.2019 20:00
What happens to atoms and chemical bonds during a reaction?
Answers: 1

Physics, 22.06.2019 04:00
Several mountains together form a mountain . a few of these combined form a mountain system. several systems combined form a mountain , which can stretch thousands of miles in length.
Answers: 2

Physics, 22.06.2019 12:10
Does anyone have the answers to online physics course plato course physics, semester a v3.0
Answers: 2

Physics, 22.06.2019 19:30
Coal contains energy. a. light b. kinetic c. chemical d. mechanical
Answers: 1
You know the right answer?
A31.15−g stainless steel ball bearing at 103.27°c is placed in a constant-pressure calorimeter conta...
Questions

Geography, 09.11.2020 18:50


Biology, 09.11.2020 18:50

Biology, 09.11.2020 18:50

Physics, 09.11.2020 18:50


History, 09.11.2020 18:50


Mathematics, 09.11.2020 18:50

Mathematics, 09.11.2020 18:50

Mathematics, 09.11.2020 18:50


Physics, 09.11.2020 18:50

English, 09.11.2020 18:50


Mathematics, 09.11.2020 18:50

Chemistry, 09.11.2020 18:50


Chemistry, 09.11.2020 18:50

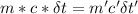
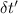 = change in temperature of water = t-22.59
= change in temperature of water = t-22.59

