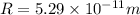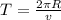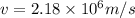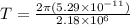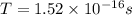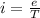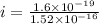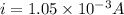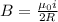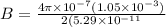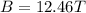Physics, 24.07.2019 16:10 itaheart101
In a simple picture of the hydrogen atom, the electron moves in circular orbits around the central proton attracted by the coulomb force. the lowest (n = 1) energy orbit that is allowed for the electron is at a radius of 5.29 × 10–11 m . calculate the magnetic field strength at the proton due to the orbital motion of the electron in the n = 1 state.

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:20
Question 11 of 13 (1 point) jump to question: a stairway, ladder, or ramp must be present in excavations in which of the following situations? a. all trenches must have access/egress b. the trench is more than 15 feet wide but only 1 ½ feet deep c. the trench is more than 4 feet deep and the devices must be within 25 feet of all workers
Answers: 1

Physics, 22.06.2019 16:50
Which best describes the first law of thermodynamics as compared to the second law of thermodynamics? a. the first law describes how thermal energy is conserved but not the direction it moves. b. the first law describes the direction thermal energy moves but not how it is conserved. c. the first law describes how thermal energy can be created but not how it can be destroyed. d. the first law describes how thermal energy can be destroyed but not how it can be created.
Answers: 1

Physics, 22.06.2019 17:00
If you wanted to move an electron from the positive to the negative terminal of the battery, how much work w would you need to do on the electron? enter your answer numerically in joules.
Answers: 1

Physics, 22.06.2019 22:10
M1 = 2.8 kg, m2 = 6.72 kg, m3 = 11.2 kg, byas in .is ,is .toof m3 it 0.91 m. (in m/s)
Answers: 3
You know the right answer?
In a simple picture of the hydrogen atom, the electron moves in circular orbits around the central p...
Questions


History, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50


Mathematics, 24.02.2021 19:50

History, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50




History, 24.02.2021 19:50




English, 24.02.2021 19:50

Social Studies, 24.02.2021 19:50

English, 24.02.2021 19:50

Mathematics, 24.02.2021 19:50