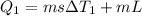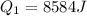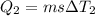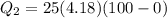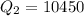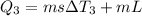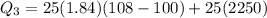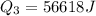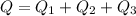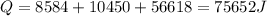Physics, 25.07.2019 02:20 ayoismeisjjjjuan
Calculate the enthalpy change (in kj) associated with the conversion of 25.0 grams of ice at -4.00 °c to water vapor at 108.0 °c. the specific heats of ice, water, and steam are 2.09 j/g-k, 4.18 j/g-k, and 1.84 j/g-k, respectively. for h2o, δ hfus

Answers: 1


Another question on Physics

Physics, 22.06.2019 13:50
The magnitude of the poynting vector of a planar electromagnetic wave has an average value of 0.939 w/m^2 . the wave is incident upon a rectangular area, 1.5 m by 2.0 m, at right angles. how much total electromagnetic energy falls on the area during 1.0 minute?
Answers: 2

Physics, 22.06.2019 15:00
What happens when a rubber rod is rubbed with a piece of fur?
Answers: 1

Physics, 22.06.2019 15:40
If an electric circuit is not grounded it is best to reach out and touch it to provide the ground a.true or b.false
Answers: 2

Physics, 22.06.2019 19:20
The dipole moment of the water molecule (h2o) is 6.17x10^-30 c.m. consider a water molecule located at the origin whose dipole moment p points in the +x-direction. a chlorine ion ( of charge-1.60x10^-19c , is located at x=3.00x10^-9m . assume that is much larger than the separation d between the charges in the dipole, so that the approximate expression for the electric field along the dipole axis can be used. a) find the magnitude of the electric force that the water molecule exerts on the chlorine ion. b) what is the direction of the electric force. -x-direction or +x-direction c) is this force attractive or repulsive?
Answers: 1
You know the right answer?
Calculate the enthalpy change (in kj) associated with the conversion of 25.0 grams of ice at -4.00 °...
Questions

Mathematics, 08.12.2019 13:31

Mathematics, 08.12.2019 13:31


Biology, 08.12.2019 13:31





History, 08.12.2019 13:31

History, 08.12.2019 13:31

Mathematics, 08.12.2019 13:31


English, 08.12.2019 13:31

History, 08.12.2019 13:31




History, 08.12.2019 13:31