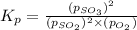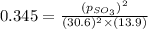Physics, 25.07.2019 05:20 diamondk2019
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2(g)+o2(g)→2so3(g) at equilibrium, the partial pressure of so2 is 30.6 atm and that of o2 is 13.9 atm. the partial pressure of so3 is atm.

Answers: 3


Another question on Physics

Physics, 22.06.2019 19:20
Aproduction line at v. j. sugumaran's machine shop has three stations. the first station can process a unit in 7 minutes. the second station has two identical machines, each of which can process a unit in 16 minutes (each unit only needs to be processed on one of the two machines). the third station can process a unit in 10 minutes. ▼ is the bottleneck station, with a bottleneck time of nothing minutes per unit (enter your response as a whole number).
Answers: 3

Physics, 22.06.2019 20:00
Anika asks eva to roll a basketball and then a bowling ball to her. which requires more force to roll, and why?
Answers: 3

Physics, 23.06.2019 00:20
What efficiency of a car's engine when heat input is 200,000 joules and waste heat is 150,000 joules? a. 75% b. 35% c. 25% d. 85%
Answers: 2

Physics, 23.06.2019 01:00
The bubbles in a carbonated soft drink are produced when carbonic acid decomposes to form carbon dioxide and water. in a closed system, this equilibrium exists as why does a carbonated soft drink lose carbonation when the container is left open? a.water evaporates, which favors the formation of h2co3. b.pressure decreases, which favors the formation of h2co3. c.the open container warms up, which causes more gas to escape. d.co2is removed, which favors the formation of h2co3.
Answers: 2
You know the right answer?
At 900.0 k, the equilibrium constant (kp) for the following reaction is 0.345. 2so2(g)+o2(g)→2so3(g)...
Questions



English, 04.06.2020 13:03

History, 04.06.2020 13:03

Mathematics, 04.06.2020 13:03



Mathematics, 04.06.2020 13:03

English, 04.06.2020 13:03

Mathematics, 04.06.2020 13:03

Mathematics, 04.06.2020 13:03

Mathematics, 04.06.2020 13:03

Mathematics, 04.06.2020 13:03

Computers and Technology, 04.06.2020 13:03


English, 04.06.2020 13:03


Mathematics, 04.06.2020 13:03

Mathematics, 04.06.2020 13:03

 is, 67.009 atm
is, 67.009 atm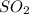 at equilibrium = 30.6 atm
at equilibrium = 30.6 atm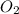 at equilibrium = 13.9 atm
at equilibrium = 13.9 atm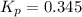
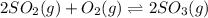
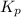 will be,
will be,