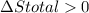Ahot object and a cold object are placed in thermal contact and the combination is isolated. they transfer energy until they reach a common temperature. the change δsh in the entropy of the hot object, the change δsc in the entropy of the cold object, and the change δstotal in the entropy of the combination are:
a) δsh > 0, δsc > 0, δstotal > 0 b) δsh < 0, δsc > 0, δstotal > 0 c) δsh < 0, δsc > 0, δstotal < 0 d) δsh > 0, δsc < 0, δstotal > 0 e) δsh > 0, δsc < 0, δstotal < 0

Answers: 3


Another question on Physics

Physics, 22.06.2019 18:00
Consider an ideal gas at 27.0 degrees celsius and 1.00 atmosphere pressure. imagine the molecules to be uniformly spaced, with each molecule at the center of a small cube. what is the length l of an edge of each small cube if adjacent cubes touch but don't overlap?
Answers: 2

Physics, 22.06.2019 20:00
Using the free-body diagram, calculate the net force acting on the sled. is the sled in a state of dynamic equilibrium?
Answers: 3

Physics, 23.06.2019 03:30
First to answer will be the brainliest i need the answer asap
Answers: 1

Physics, 23.06.2019 08:30
[05.02] what is the best explanation of the kinetic molecular theory as it relates to the energy of the molecules in the states of matter? (1 point) the molecules in a gas have greater kinetic energy than those in a liquid, which in turn have greater kinetic energy than the molecules in a solid. kinetic energy cannot be created nor destroyed, and, as a result, all states of matter have the same kinetic energy. the molecules in a gas have greater kinetic energy; therefore, they are more tightly packed than the molecules in either a liquid or a solid. the molecules in gases and liquids, in constant motion, have greater kinetic energy than those in a solid, which do not move or vibrate because their position is fixed.
Answers: 2
You know the right answer?
Ahot object and a cold object are placed in thermal contact and the combination is isolated. they tr...
Questions





Mathematics, 14.12.2019 07:31

Mathematics, 14.12.2019 07:31

Chemistry, 14.12.2019 07:31



Computers and Technology, 14.12.2019 07:31



English, 14.12.2019 07:31

English, 14.12.2019 07:31




English, 14.12.2019 07:31

Mathematics, 14.12.2019 07:31

 ,
,  ,
,