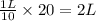Astudent is told to use 20.0 g of sodium chloride to make an aqueous solution that has a concentration of 10.0 g/l (grams of sodium chloride per liter of solution). assuming that 20.0 g of sodium chloride has a volume of 7.50 ml, show that she will need about 1.99 l of water to make this solution. in making this solution, should she add the solute to the solvent or the solvent to the solute?

Answers: 2


Another question on Physics

Physics, 22.06.2019 09:10
Which lists the organs in the correct order as food passes from the mouth to anus?
Answers: 1

Physics, 22.06.2019 09:40
(a) assume the equation x = at^3 + bt describes the motion of a particular object, with x having the dimension of length and t having the dimension of time. determine the dimensions of the constants a and b. (use the following as necessary: l and t, where l is the unit of length and t is the unit of time.) (b) determine the dimensions of the derivative dx/dt = 3at^2 + b. (use the following as necessary: l and t, where l is the unit of length and t is the unit of time.)
Answers: 1


You know the right answer?
Astudent is told to use 20.0 g of sodium chloride to make an aqueous solution that has a concentrati...
Questions

Mathematics, 29.06.2019 15:30

Mathematics, 29.06.2019 15:30

English, 29.06.2019 15:30


Mathematics, 29.06.2019 15:30



Mathematics, 29.06.2019 15:30


History, 29.06.2019 15:30

Mathematics, 29.06.2019 15:30


Mathematics, 29.06.2019 15:30

Biology, 29.06.2019 15:30

History, 29.06.2019 15:30

Physics, 29.06.2019 15:30