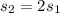Physics, 31.07.2019 20:30 olivialaine31
Athermally isolated system is made up of a hot piece of aluminum and a cold piece of copper; the aluminum and the copper are in thermal contact. the specific heat capacity of aluminum is more than double that of copper. which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?

Answers: 3


Another question on Physics

Physics, 22.06.2019 08:20
A3030 cmcm wrench is used to loosen a bolt with a force applied 0.30.3 mm from the bolt. it takes 6060 nn to loosen the bolt when the force is applied perpendicular to the wrench. how much force would it take if the force was applied at a 3030 degree angle from perpendicular? a 3030 cmcm wrench is used to loosen a bolt with a force applied 0.30.3 mm from the bolt. it takes 6060 nn to loosen the bolt when the force is applied perpendicular to the wrench. how much force would it take if the force was applied at a 3030 degree angle from perpendicular?
Answers: 3

Physics, 22.06.2019 11:00
Aperson walks first at a constant speed of 4.89 m/s along a straight line from point a to point b and then back along the line from b to a at a constant speed of 2.95 m/s. what is the average speed over the entire trip?
Answers: 1

Physics, 22.06.2019 15:30
Charge is distributed along the entire x-axis with uniform density λ. how much work does the electric field of this charge distribution do on an electron that moves along the y-axis from y = a to y = b? (use the following as necessary: a, b, ε0, λ, and q for the charge on an electron.)
Answers: 3

Physics, 22.06.2019 17:50
If there's a small amount of friction between two surfaces, the result could be select all that applya. no movement b. heatc. a little bit of movementd. sliding around
Answers: 2
You know the right answer?
Athermally isolated system is made up of a hot piece of aluminum and a cold piece of copper; the al...
Questions



History, 23.11.2019 15:31

World Languages, 23.11.2019 15:31

History, 23.11.2019 15:31

History, 23.11.2019 15:31

History, 23.11.2019 15:31


Mathematics, 23.11.2019 15:31



Mathematics, 23.11.2019 15:31

Advanced Placement (AP), 23.11.2019 15:31

Chemistry, 23.11.2019 15:31

Physics, 23.11.2019 15:31

Mathematics, 23.11.2019 15:31


History, 23.11.2019 15:31


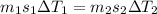
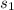 = specific heat capacity of copper
= specific heat capacity of copper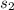 = specific heat capacity of aluminum
= specific heat capacity of aluminum