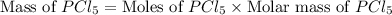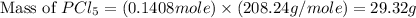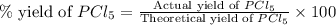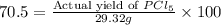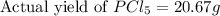Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of product produced when 25.0 g of phosphorous reacts with 25.0 grams of chlorine. calculate the mass of product produced if the reaction occurred with a 70.5 percent yield.

Answers: 1


Another question on Physics

Physics, 21.06.2019 23:40
Aregular polygon has angkes of size 150° each.how many side has the polygon
Answers: 1

Physics, 22.06.2019 05:30
Could someone . i have tried to solve myself but my calculation is off
Answers: 3

Physics, 22.06.2019 11:00
What is the measurement of how fast an object moves relative to a reference point?
Answers: 1

You know the right answer?
Phosphorous reacts with chlorine gas to produce phosphorous pentachloride. calculate the mass of pro...
Questions


Mathematics, 06.05.2020 07:15






History, 06.05.2020 07:15



History, 06.05.2020 07:15









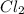 = 25 g
= 25 g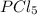 = 208.24 g/mole
= 208.24 g/mole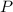 and
and 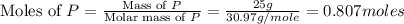
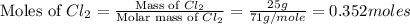
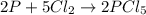
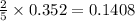 moles of
moles of