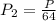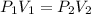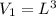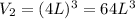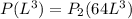An ideal gas in a cubical box having sides of length l exerts a pressure p on the walls of the box. if all of this gas is put into a box having sides of length 4l without changing its temperature, the pressure it exerts on the walls of the larger box will be
a. 1/16 p
b. p
c. 1/64 p
d. 1/4 p

Answers: 3


Another question on Physics

Physics, 22.06.2019 14:30
Which of the following bonds would be most polar? a. c-i b. c-br c. c-cl d. c-f e. c-o
Answers: 1

Physics, 22.06.2019 19:20
On a hot saturday morning while people are working inside, the air conditioner keeps the temperature inside the building at 22degreesc. at noon the air conditioner is turned off, and the people go home. the temperature outside is a constant 33degreesc for the rest of the afternoon. if the time constant for the building is 4 hr, what will be the temperature inside the building at 4 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? at 6 : 00 font size decreased by 2 upper p . font size decreased by 2 upper m .? when will the temperature inside the building reach 24degreesc? 324281
Answers: 3

Physics, 22.06.2019 22:00
Aviolently rotating column of air is formed around a region of extremely low air pressure above land. which of these weather conditions is most likely to follow? blizzard cyclone tornado hurricane
Answers: 1

Physics, 22.06.2019 23:30
An electric power distributor charges residential customers $0.10 per kilowatt-hour (kwh). the company advertises that "green power" is available in 150 kwh blocks for an additional $4 per month. (green power is generated from solar, wind power, and methane sources.) if a certain customer uses an average of 391 kwh per month and commits to one monthly 150 kwh block of green power, what is her annual power bill
Answers: 1
You know the right answer?
An ideal gas in a cubical box having sides of length l exerts a pressure p on the walls of the box....
Questions




English, 27.09.2021 06:50


English, 27.09.2021 06:50

Mathematics, 27.09.2021 06:50













Mathematics, 27.09.2021 06:50