Physics, 01.08.2019 00:30 jeanneschu
A2.00-kg block of aluminum at 50.0 °c is dropped into 5.00 kg of water at 20.0 °c. what is the change in entropy during the approach to equilibrium, assuming no heat is exchanged with the environment? the specific heat of aluminum is 0.22 cal/(g∙k).

Answers: 1


Another question on Physics

Physics, 21.06.2019 19:30
Act has a 1200: 5 or 240: 1 turns ratio. it is short circuited. what is the maximum current at 1200a primary if we put a 100ohm resistor across the secondary end of the ct. use it as current source circuit.
Answers: 2

Physics, 22.06.2019 06:30
Organelles are 1. responsible for producing power for the cell 2. tiny structures in the cell that carry out the cell's activities 3. responsible for digestion in the cell 4. found outside of the membrane
Answers: 1

Physics, 22.06.2019 13:00
Discuss how the hardness or softness of the landing surface is related to the time required to stop the egg
Answers: 1

Physics, 22.06.2019 13:00
What effects result when there is an impact between earth and an asteroid? check all that apply. a.massive flooding. b.extinction of many life forms.c.major earthquakes change in average global temperature.d. giant dust cloud
Answers: 3
You know the right answer?
A2.00-kg block of aluminum at 50.0 °c is dropped into 5.00 kg of water at 20.0 °c. what is the chang...
Questions




Mathematics, 13.01.2021 23:00







Mathematics, 13.01.2021 23:00

Mathematics, 13.01.2021 23:00




Mathematics, 13.01.2021 23:00


Mathematics, 13.01.2021 23:00

History, 13.01.2021 23:00

Mathematics, 13.01.2021 23:00

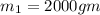
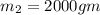
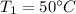 = 323 K
= 323 K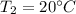 = 293 K
= 293 K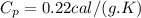
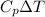
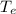 ' K
' K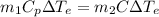
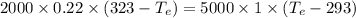
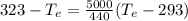
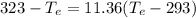
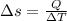
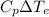
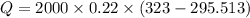 = 12094.28 cal
= 12094.28 cal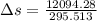 = 40.93 cal/K = 9.78 J/K
= 40.93 cal/K = 9.78 J/K