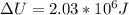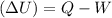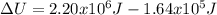Physics, 03.08.2019 06:10 leoiscoolcool
When water is boiled under a pressure of 2.00 atm, the heat of vaporization is 2.20×10^6 j/kg and the boiling point is 120 c. at this pressure, 1.00 kg of water has a volume of 1.00×10^−3 m3, and 1.00 kg of steam has a volume of 0.824 m^3.(a)compute the work done when 1.00kg of steam is formed at this temperature(b)compute the increase in internal energy of the water.

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:50
You are given a vector a = 125i and an unknown vector b that is perpendicular to a. the cross-product of these two vectors is a × b = 98k. what is the y-component of vector b?
Answers: 1

Physics, 22.06.2019 15:50
Ryan is examining the energy of the particles in a bar of gold. what is ryan most likely studying?
Answers: 2

Physics, 23.06.2019 02:20
Aflute player hears four beats per second when she compares her note to a 523 hz tuning fork (the note c). she can match the frequency of the tuning fork by pulling out the “tuning join” to lengthen her flute slightly. what was her initial frequency?
Answers: 2

Physics, 23.06.2019 05:00
Scientists discovered fossils in several layers of the earth you see here. they found fossils of algae, snails, and clams in layer d. given that information, where do you think they found fossil evidence of simple land plants and amphibians?
Answers: 2
You know the right answer?
When water is boiled under a pressure of 2.00 atm, the heat of vaporization is 2.20×10^6 j/kg and th...
Questions


Mathematics, 05.02.2021 18:20


Social Studies, 05.02.2021 18:20

Mathematics, 05.02.2021 18:20


Mathematics, 05.02.2021 18:20


History, 05.02.2021 18:20

Chemistry, 05.02.2021 18:20

Mathematics, 05.02.2021 18:20


History, 05.02.2021 18:20


Mathematics, 05.02.2021 18:20

Biology, 05.02.2021 18:20

History, 05.02.2021 18:20


Mathematics, 05.02.2021 18:20