Physics, 05.08.2019 16:10 dejaproctor17
To find the specific heat capacity of a certain metal, a student places a block of the metal that weighs 27 grams and has an initial temperature of 97 deg c, into 52 grams of water with a temperature of 20 deg c. the final temperature was measured to be 27 deg c. what is the heat capacity of the metal?

Answers: 1


Another question on Physics

Physics, 22.06.2019 03:00
Which of the following harmful chemicals are found in tobacco smoke? a. carbon monoxide b. carbon dioxide c. nicotine b. carbon dioxide d. both a and c
Answers: 2

Physics, 22.06.2019 10:10
Which branches of natural science include the study of an organism that lived 10 million years ago
Answers: 1

Physics, 22.06.2019 18:50
An insulated thermos contains 148 g of water at 72.7 ˚c. you put in a 11.7 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?
Answers: 2

Physics, 22.06.2019 22:00
Which of these is the best way to manage a natural resource
Answers: 3
You know the right answer?
To find the specific heat capacity of a certain metal, a student places a block of the metal that we...
Questions


English, 01.12.2020 19:10


Mathematics, 01.12.2020 19:10

Mathematics, 01.12.2020 19:10

Spanish, 01.12.2020 19:10



Arts, 01.12.2020 19:10


Biology, 01.12.2020 19:10



Arts, 01.12.2020 19:10

Spanish, 01.12.2020 19:10

Mathematics, 01.12.2020 19:10




English, 01.12.2020 19:10

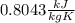
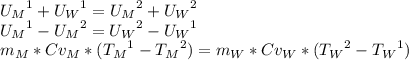
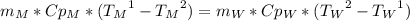
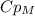 , so:
, so: