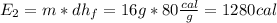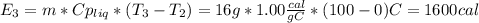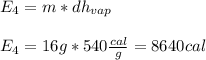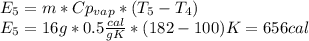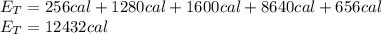Physics, 05.08.2019 16:10 ayoismeisalex
16 grams of ice at –32°c is to be changed to steam at 182°c. the entire process requires cal. round your answer to the nearest whole number. the specific heat of both ice and steam is 0.5 cal/g°c. the specific heat of water is 1.00 cal/gk. the heat of fusion is 80 cal/g and the heat of vaporization is 540 cal/g.

Answers: 3


Another question on Physics


Physics, 21.06.2019 23:00
The roller coaster from problem #1 then tops a second hill at 15.0 m/s, how high is the second hill? 91.5 m 79.2 m 80.0 m 68.5 m
Answers: 1

Physics, 22.06.2019 18:00
Avector of magnitude 2 cannot be added to a vector of magnitude 3 so that the magnitude of the resultant is a. zero b. 1 c. 3 d. 5 e. 7
Answers: 1

Physics, 23.06.2019 02:40
An air standard cycle with variable specific heats is executed in a closed system and is composed of the following four processes. 1-2 isentropic compression 100 kpa and 22°c to 600 kpa 2-3 v=constant heat addition to 1500 k 3-4 isentropic expansion to 100 kpa 4-1 p=constant heat rejection to initial state (a) show the cycle on p-v and t-s s (b) calculate the net work output per unit mass (409 kj/kg) (c) determine the thermal efficiency (47.9%)
Answers: 1
You know the right answer?
16 grams of ice at –32°c is to be changed to steam at 182°c. the entire process requires cal. round...
Questions

History, 05.07.2019 10:00

Chemistry, 05.07.2019 10:00

Mathematics, 05.07.2019 10:00


History, 05.07.2019 10:00

Social Studies, 05.07.2019 10:00

Chemistry, 05.07.2019 10:00

Mathematics, 05.07.2019 10:00


English, 05.07.2019 10:00

Computers and Technology, 05.07.2019 10:00

History, 05.07.2019 10:00

Social Studies, 05.07.2019 10:00


French, 05.07.2019 10:00

Social Studies, 05.07.2019 10:00


History, 05.07.2019 10:00

Advanced Placement (AP), 05.07.2019 10:00

History, 05.07.2019 10:00

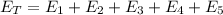
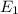 is a heating process for the ice, so we know that the energy required is proportional to the temperature difference through the specific heat:
is a heating process for the ice, so we know that the energy required is proportional to the temperature difference through the specific heat: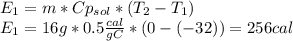
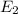 is a phase change process, so we do not use the specific heat (sensible heat), but the fusion heat (latent heat), so:
is a phase change process, so we do not use the specific heat (sensible heat), but the fusion heat (latent heat), so: