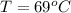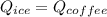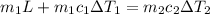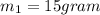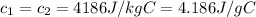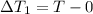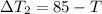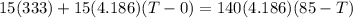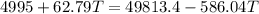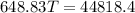An insulated thermos contains 140 cm3 of hot coffee at 85.0°c. you put in a 15.0 g ice cube at its melting point to cool the coffee. by how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? treat the coffee as though it were pure water and neglect energy exchanges with the environment. the specific heat of water is 4186 j/kg·k. the latent heat of fusion is 333 kj/kg. the density of water is 1.00 g/cm3.

Answers: 2


Another question on Physics

Physics, 21.06.2019 21:30
One day, after pulling down your window shade, you notice that sunlight is passing through a pinhole in the shade and making a small patch of light on the far wall. you see that the patch of light seems to be a circular diffraction pattern. it appears that the central maximum is about 1 cm across and you estimate that the distance from the window shade to the wall is about 3 m. what is (a) the average wavelength of sunlight? (b) the diameter of the pinhole?
Answers: 3

Physics, 21.06.2019 23:00
In a given chemical reaction, the energy of the products is greater than the energy of the reactants. which statement is true for this reaction?
Answers: 2

Physics, 22.06.2019 03:30
Calculate the mass of an object that has a momentum of 100kg x m/sec and velocity of 4 m/sec
Answers: 1

You know the right answer?
An insulated thermos contains 140 cm3 of hot coffee at 85.0°c. you put in a 15.0 g ice cube at its m...
Questions

History, 01.03.2021 16:50



Mathematics, 01.03.2021 16:50


Mathematics, 01.03.2021 16:50


English, 01.03.2021 16:50

English, 01.03.2021 16:50


Computers and Technology, 01.03.2021 16:50





History, 01.03.2021 16:50


Mathematics, 01.03.2021 16:50

Computers and Technology, 01.03.2021 16:50