Physics, 30.08.2019 23:30 irenecupcake3467
Alaboratory technician drops a 72.0 g sample of unknown solid material, at a temperature of 80.0°c, into a calorimeter. the calorimeter can, initially at 11.0°c, is made of 187 g of copper and contains 203 g of water. the final temperature of the calorimeter can and contents is 39.4°c. what is the specific heat of the unknown sample? give your answer in units of j/kg.°c.

Answers: 3


Another question on Physics

Physics, 21.06.2019 18:30
Will a heavier bowling ball hit pins better than a lighter bsll
Answers: 2

Physics, 22.06.2019 03:00
If it takes a planet 2.8 x 10^8 s to orbit a star with a mass of 6.2 x 10^30 kg what is the average distance between the planet and the star
Answers: 3

Physics, 22.06.2019 17:40
You throw a baseball directly upward at time =0 at an initial speed of 14.9 m/s. what is the maximum height the ball reaches above where it leaves your hand? ignore air resistance and take =9.80 m/s2.
Answers: 1

Physics, 22.06.2019 20:30
Why does karst topography exist in some geographic location? a. few climates have enough oxygen b. most regions have rock layers other than limestone c. in most regions, the soil is too thin d. the water is too acidic enough in most places
Answers: 2
You know the right answer?
Alaboratory technician drops a 72.0 g sample of unknown solid material, at a temperature of 80.0°c,...
Questions

English, 18.10.2020 21:01




History, 18.10.2020 21:01

Chemistry, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01


Business, 18.10.2020 21:01





Chemistry, 18.10.2020 21:01

Mathematics, 18.10.2020 21:01



Mathematics, 18.10.2020 21:01


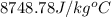
![q_1=-[q_2+q_3]](/tpl/images/0213/3390/74013.png)
![m_1\times c_1\times (T_f-T_1)=-[m_2\times c_2\times (T_f-T_2)+m_3\times c_3\times (T_f-T_2)]](/tpl/images/0213/3390/411ef.png)
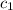 = specific heat of unknown sample = ?
= specific heat of unknown sample = ?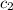 = specific heat of water =
= specific heat of water = 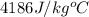
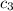 = specific heat of copper =
= specific heat of copper = 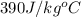
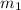 = mass of unknown sample = 72.0 g = 0.072 kg
= mass of unknown sample = 72.0 g = 0.072 kg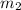 = mass of water = 203 g = 0.203 kg
= mass of water = 203 g = 0.203 kg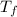 = final temperature of calorimeter =
= final temperature of calorimeter = 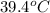
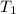 = initial temperature of unknown sample =
= initial temperature of unknown sample = 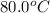
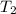 = initial temperature of water and copper =
= initial temperature of water and copper = 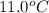
![0.072kg\times c_1\times (39.4-80.0)^oC=-[(0.203kg\times 4186J/kg^oC\times (39.4-11.0)^oC)+(0.187kg\times 390J/kg^oC\times (39.4-11.0)^oC)]](/tpl/images/0213/3390/83692.png)